Diagnostic Yield From 339 Epilepsy Patients Screened on a Clinical Gene Panel
- PMID: 29056246
- PMCID: PMC6885003
- DOI: 10.1016/j.pediatrneurol.2017.09.003
Diagnostic Yield From 339 Epilepsy Patients Screened on a Clinical Gene Panel
Abstract
Background: The contribution of genetic factors to epilepsy has long been recognized and has been estimated to play a role in 70% to 80% of cases. Identification of a pathogenic variant can help families to better cope with the disorder, allows for genetic counseling to determine recurrence risk, and in some cases, can directly influence treatment options. In this study, we determined the diagnostic yield of a clinical gene panel applied to an unselected cohort of epilepsy patients.
Methods: Variant reports from 339 clinically referred epilepsy patients screened using a 110-gene panel were retrospectively reviewed. Variants were classified using the American College of Medical Genetics and Genomics guidelines.
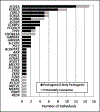
Results: Pathogenic or likely pathogenic variants were identified in 62 individuals (18%) and potentially causative variants were identified in an additional 21 individuals (6%). Causative and potentially causative variants were most frequently identified in SCN1A (n = 15) and KCNQ2 (n = 10). Other genes in which disease-causing variants were identified in multiple individuals included CDKL5, SCN2A, SCN8A, SCN1B, STXBP1, TPP1, PCDH19, CACNA1A, GABRA1, GRIN2A, SLC2A1, and TSC2. Sixteen additional genes had variants identified in single individuals.
Conclusions: We identified 87 variants in 30 different genes that could explain disease, of which 54% were not previously reported. This study confirms the utility of targeted gene panel analysis in epilepsy and highlights several factors to improve the yield of diagnostic genetic testing, including the critical need for clinical phenotype information and parental samples, microarray analysis for whole exon deletions and duplications, and frequent update of panels to incorporate new disease genes.
Keywords: diagnostic yield; epilepsy; epileptic encephalopathy; genetic testing.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTERESTS
CdS and JJA are employed by and receive a salary from EGL Genetics. The Epilepsy and Seizure Disorders panel is among EGL Genetics’ commercially available tests. MH is a former employee of EGL Genetics. KMB and AE have no conflicts of interest.
Figures
References
-
- Wang J, Lin ZJ, Liu L, et al. Epilepsy-associated genes. Seizure. 2017;44:11–20. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous