Apolipoprotein E as a novel therapeutic neuroprotection target after traumatic spinal cord injury
- PMID: 29056364
- PMCID: PMC5967384
- DOI: 10.1016/j.expneurol.2017.10.014
Apolipoprotein E as a novel therapeutic neuroprotection target after traumatic spinal cord injury
Abstract
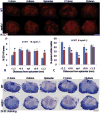
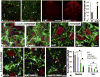
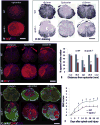
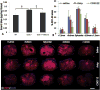
Apolipoprotein E (apoE), a plasma lipoprotein well known for its important role in lipid and cholesterol metabolism, has also been implicated in many neurological diseases. In this study, we examined the effect of apoE on the pathophysiology of traumatic spinal cord injury (SCI). ApoE-deficient mutant (apoE-/-) and wild-type mice received a T9 moderate contusion SCI and were evaluated using histological and behavioral analyses after injury. At 3days after injury, the permeability of spinal cord-blood-barrier, measured by extravasation of Evans blue dye, was significantly increased in apoE-/- mice compared to wild type. The inflammation and spared white matter was also significantly increased and decreased, respectively, in apoE-/- mice compared to the wild type ones. The apoptosis of both neurons and oligodendrocytes was also significantly increased in apoE-/- mice. At 42days after injury, the inflammation was still robust in the injured spinal cord in apoE-/- but not wild type mice. CD45+ leukocytes from peripheral blood persisted in the injured spinal cord of apoE-/- mice. The spared white matter was significantly decreased in apoE-/- mice compared to wild type ones. Locomotor function was significantly decreased in apoE-/- mice compared to wild type ones from week 1 to week 8 after contusion. Treatment of exogenous apoE mimetic peptides partially restored the permeability of spinal cord-blood-barrier in apoE-/- mice after SCI. Importantly, the exogenous apoE peptides decreased inflammation, increased spared white matter and promoted locomotor recovery in apoE-/- mice after SCI. Our results indicate that endogenous apoE plays important roles in maintaining the spinal cord-blood-barrier and decreasing inflammation and spinal cord tissue loss after SCI, suggesting its important neuroprotective function after SCI. Our results further suggest that exogenous apoE mimetic peptides could be a novel and promising neuroprotective reagent for SCI.
Keywords: Apolipoprotein E; Neuroprotection; Spinal cord injury; Spinal cord-blood-barrier; Treatment.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, Roses AD. ApoE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346:575. - PubMed
-
- Aono M, Bennett ER, Kim KS, Lynch JR, Myers J, Pearlstein RD, Warner DS, Laskowitz DT. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-d-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–445. - PubMed
-
- Bart RD, Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Regional CBF in apolipoprotein E-deficient and wild type mice during focal cerebral ischemia. Neuroreport. 1998;9:2615–2620. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

