A Highly Pathogenic Avian H7N9 Influenza Virus Isolated from A Human Is Lethal in Some Ferrets Infected via Respiratory Droplets
- PMID: 29056430
- PMCID: PMC5721358
- DOI: 10.1016/j.chom.2017.09.008
A Highly Pathogenic Avian H7N9 Influenza Virus Isolated from A Human Is Lethal in Some Ferrets Infected via Respiratory Droplets
Abstract
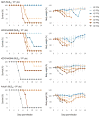
Low pathogenic H7N9 influenza viruses have recently evolved to become highly pathogenic, raising concerns of a pandemic, particularly if these viruses acquire efficient human-to-human transmissibility. We compared a low pathogenic H7N9 virus with a highly pathogenic isolate, and two of its variants that represent neuraminidase inhibitor-sensitive and -resistant subpopulations detected within the isolate. The highly pathogenic H7N9 viruses replicated efficiently in mice, ferrets, and/or nonhuman primates, and were more pathogenic in mice and ferrets than the low pathogenic H7N9 virus, with the exception of the neuraminidase inhibitor-resistant virus, which showed mild-to-moderate attenuation. All viruses transmitted among ferrets via respiratory droplets, and the neuraminidase-sensitive variant killed several of the infected and exposed animals. Neuraminidase inhibitors showed limited effectiveness against these viruses in vivo, but the viruses were susceptible to a polymerase inhibitor. These results suggest that the highly pathogenic H7N9 virus has pandemic potential and should be closely monitored.
Keywords: antiviral sensitivity; ferrets; highly pathogenic avian influenza H7N9 viruses; mice; nonhuman primates; pathogenicity; receptor-binding specificity; replication capacity; transmissibility.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

