Discovery of Mechanism-Based Inactivators for Human Pancreatic Carboxypeptidase A from a Focused Synthetic Library
- PMID: 29057062
- PMCID: PMC5641956
- DOI: 10.1021/acsmedchemlett.7b00346
Discovery of Mechanism-Based Inactivators for Human Pancreatic Carboxypeptidase A from a Focused Synthetic Library
Abstract
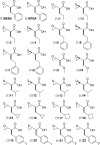
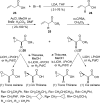
Metallocarboxypeptidases (MCPs) are involved in many biological processes such as fibrinolysis or inflammation, development, Alzheimer's disease, and various types of cancer. We describe the synthesis and kinetic characterization of a focused library of 22 thiirane- and oxirane-based potential mechanism-based inhibitors, which led to discovery of an inhibitor for the human pro-carboxypeptidase A1. Our structural analyses show that the thiirane-based small-molecule inhibitor penetrates the barrier of the pro-domain to bind within the active site. This binding leads to a chemical reaction that covalently modifies the catalytic Glu270. These results highlight the importance of combined structural, biophysical, and biochemical evaluation of inhibitors in design strategies for the development of spectroscopically nonsilent probes as effective beacons for in vitro, in cellulo, and/or in vivo localization in clinical and industrial applications.
Keywords: Carboxypeptidase A; X-ray crystallography; mechanism-based inactivators; thiiranes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

