Fluorogenic reaction-based prodrug conjugates as targeted cancer theranostics
- PMID: 29057403
- PMCID: PMC5750141
- DOI: 10.1039/c7cs00557a
Fluorogenic reaction-based prodrug conjugates as targeted cancer theranostics
Abstract
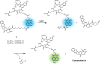
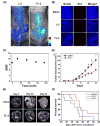
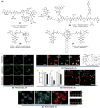
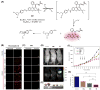
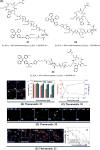
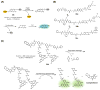
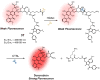
Theranostic systems are receiving ever-increasing attention due to their potential therapeutic utility, imaging enhancement capability, and promise for advancing the field of personalized medicine, particularly as it relates to the diagnosis, staging, and treatment of cancer. In this Tutorial Review, we provide an introduction to the concepts of theranostic drug delivery effected via use of conjugates that are able to target cancer cells selectively, provide cytotoxic chemotherapeutics, and produce readily monitored imaging signals in vitro and in vivo. The underlying design concepts, requiring the synthesis of conjugates composed of imaging reporters, masked chemotherapeutic drugs, cleavable linkers, and cancer targeting ligands, are discussed. Particular emphasis is placed on highlighting the potential benefits of fluorogenic reaction-based targeted systems that are activated for both imaging and therapy by cellular entities, e.g., thiols, reactive oxygen species and enzymes, which are present at relatively elevated levels in tumour environments, physiological characteristics of cancer, e.g., hypoxia and acidic pH. Also discussed are systems activated by an external stimulus, such as light. The work summarized in this Tutorial Review will help define the role fluorogenic reaction-based, cancer-targeting theranostics may have in advancing drug discovery efforts, as well as improving our understanding of cellular uptake and drug release mechanisms.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









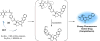

References
-
- Kumar R, Shin WS, Sunwoo K, Kim WY, Koo S, Bhuniya S, Kim JS. Chem. Soc. Rev. 2015;44:6670. - PubMed
-
- Lim EK, Kim T, Paik S, Haam S, Huh Y-M, Lee K. Chem. Rev. 2015;115:327. - PubMed
-
- Weinstain R, Segal E, Satchi-Fainaro R, Shabat D. Chem. Commun. 2010;46:553. - PubMed
-
- Lee MH, Sessler JL, Kim JS. Acc. Chem. Res. 2015;48:2935. - PubMed
-
- Gnaim S, Shabat D. Acc. Chem. Res. 2014;47:2970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

