Insulin-like growth factor-1 activates PI3K/Akt signalling to protect human retinal pigment epithelial cells from amiodarone-induced oxidative injury
- PMID: 29057462
- PMCID: PMC5740246
- DOI: 10.1111/bph.14078
Insulin-like growth factor-1 activates PI3K/Akt signalling to protect human retinal pigment epithelial cells from amiodarone-induced oxidative injury
Abstract
Background and purpose: Amiodarone is one of the most effective anti-arrhythmic drugs available, but its clinical applications are limited by toxic side effects including optic toxicity. The purpose of this study was to investigate the toxic effect of amiodarone on D407 cells (a human retinal pigmented epithelial (RPE) cell line) and the mechanisms of the protective effect of insulin-like growth factor-1 (IGF-1).
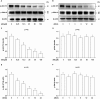
Experimental approach: The involvement of the kinases, Akt and ERK, was analysed by Western blot. Intracellular accumulation of ROS was measured using fluorophotometric quantification. A pharmacological approach with inhibitors was used to investigate the pathways involved in the protective action of IGF-1.
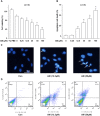
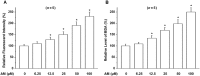
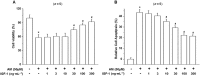
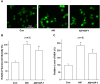
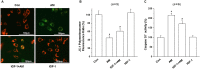
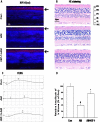
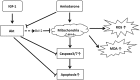
Key results: Amiodarone concentration-dependently augmented the production of ROS, lipid peroxidation and apoptosis in D407 cells. IGF-1 time- and concentration-dependently reversed these effects of amiodarone and protected D407 cells from amiodarone-mediated toxicity. Amiodarone inhibited the pAkt but not pErk, and IGF-1 reversed this inhibitory effect of amiodarone. However, IGF-1 failed to suppress amiodarone-induced cytotoxicity in the presence of PI3K/Akt inhibitor LY294002 suggesting the direct involvement of the PI3K/Akt pathway. Furthermore, in vivo rat flash electroretinogram (FERG) recordings showed that IGF-1 reverses the amiodarone-induced decrease in a- and b-waves. The immunocytochemistry findings confirmed that vitreous IGF-1 injections promote the survival of RPE cells in rat retina treated with amiodarone.
Conclusion and implications: IGF-1 can protect RPE cells from amiodarone-mediated injury via the PI3K/Akt pathway in vivo and in vitro. IGF-1 has potential as a protective drug for the prevention and treatment of amiodarone-induced optic toxicity.
© 2017 The British Pharmacological Society.
Figures


Similar articles
-
Amiodarone-Induced Retinal Neuronal Cell Apoptosis Attenuated by IGF-1 via Counter Regulation of the PI3k/Akt/FoxO3a Pathway.Mol Neurobiol. 2017 Nov;54(9):6931-6943. doi: 10.1007/s12035-016-0211-x. Epub 2016 Oct 24. Mol Neurobiol. 2017. PMID: 27774572
-
IGF-1-Mediated Survival from Induced Death of Human Primary Cultured Retinal Pigment Epithelial Cells Is Mediated by an Akt-Dependent Signaling Pathway.Mol Neurobiol. 2018 Mar;55(3):1915-1927. doi: 10.1007/s12035-017-0447-0. Epub 2017 Feb 25. Mol Neurobiol. 2018. PMID: 28238097
-
IGF-1 signaling via the PI3K/Akt pathway confers neuroprotection in human retinal pigment epithelial cells exposed to sodium nitroprusside insult.J Mol Neurosci. 2015 Apr;55(4):931-40. doi: 10.1007/s12031-014-0448-7. Epub 2014 Oct 23. J Mol Neurosci. 2015. PMID: 25339505
-
Clinical and Mechanistic Review of Amiodarone-Associated Optic Neuropathy.Biomolecules. 2022 Sep 14;12(9):1298. doi: 10.3390/biom12091298. Biomolecules. 2022. PMID: 36139137 Free PMC article. Review.
-
Oxidative stress induced cellular signaling in RPE cells.Front Biosci (Schol Ed). 2012 Jan 1;4(2):392-411. doi: 10.2741/s275. Front Biosci (Schol Ed). 2012. PMID: 22202067 Review.
Cited by
-
Amiodarone Advances the Apoptosis of Cardiomyocytes by Repressing Sigmar1 Expression and Blocking KCNH2-related Potassium Channels.Curr Mol Med. 2025;25(1):69-78. doi: 10.2174/0115665240265771231129105108. Curr Mol Med. 2025. PMID: 38204277
-
Chrysin Ameliorates Malfunction of Retinoid Visual Cycle through Blocking Activation of AGE-RAGE-ER Stress in Glucose-Stimulated Retinal Pigment Epithelial Cells and Diabetic Eyes.Nutrients. 2018 Aug 8;10(8):1046. doi: 10.3390/nu10081046. Nutrients. 2018. PMID: 30096827 Free PMC article.
-
Heparin impairs skeletal muscle glucose uptake by inhibiting insulin binding to insulin receptor.Endocrinol Diabetes Metab. 2021 May 5;4(3):e00253. doi: 10.1002/edm2.253. eCollection 2021 Jul. Endocrinol Diabetes Metab. 2021. PMID: 34277977 Free PMC article.
-
The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems.Prog Retin Eye Res. 2021 Nov;85:100969. doi: 10.1016/j.preteyeres.2021.100969. Epub 2021 Apr 23. Prog Retin Eye Res. 2021. PMID: 33901682 Free PMC article. Review.
-
Artemisinin conferred cytoprotection to human retinal pigment epithelial cells exposed to amiodarone-induced oxidative insult by activating the CaMKK2/AMPK/Nrf2 pathway.J Transl Med. 2024 Sep 16;22(1):844. doi: 10.1186/s12967-024-05593-x. J Transl Med. 2024. PMID: 39285426 Free PMC article.
References
-
- Acerini CL, Patton CM, Savage MO, Kernell A, Westphal O, Dunger DB (1997). Randomised placebo‐controlled trial of human recombinant insulin‐like growth factor I plus intensive insulin therapy in adolescents with insulin‐dependent diabetes mellitus. Lancet 350: 1199–1204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

