Predictors Associated with Increase in Skeletal Muscle Mass after Sustained Virological Response in Chronic Hepatitis C Treated with Direct Acting Antivirals
- PMID: 29057827
- PMCID: PMC5691751
- DOI: 10.3390/nu9101135
Predictors Associated with Increase in Skeletal Muscle Mass after Sustained Virological Response in Chronic Hepatitis C Treated with Direct Acting Antivirals
Abstract
Aims: We aimed to examine changes in skeletal muscle mass in chronic hepatitis C (CHC) patients undergoing interferon (IFN)-free direct acting antivirals (DAAs) therapy who achieved sustained virological response (SVR).
Patients and methods: A total of 69 CHC patients treated with DAAs were analyzed. We compared the changes in skeletal muscle index (SMI) using bio-impedance analysis at baseline and SMI at SVR. SMI was calculated as the sum of skeletal muscle mass in upper and lower extremities divided by height squared (cm²/m²). Further, we identified pretreatment parameters contributing to the increased SMI at SVR.
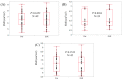
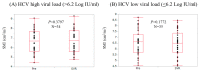
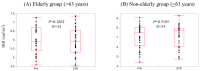
Results: SMI in males at baseline ranged from 6.73 to 9.08 cm²/m² (median, 7.65 cm²/m²), while that in females ranged from 4.45 to 7.27 cm²/m² (median, 5.81 cm²/m²). At SVR, 36 patients (52.2%) had increased SMI as compared with baseline. In the univariate analysis, age (p = 0.0392), hyaluronic acid (p = 0.0143), and branched-chain amino acid to tyrosine ratio (BTR) (p = 0.0024) were significant pretreatment factors linked to increased SMI at SVR. In the multivariate analysis, only BTR was an independent predictor linked to the increased SMI at SVR (p = 0.0488).
Conclusion: Pretreatment BTR level can be helpful for predicting increased SMI after SVR in CHC patients undergoing IFN-free DAAs therapy.
Keywords: chronic hepatitis C; direct acting antiviral; skeletal muscle mass; sustained virological response.
Conflict of interest statement
In the current study, the authors declare that they have no conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

