Stopped-Flow Fluorometric Ion Flux Assay for Ligand-Gated Ion Channel Studies
- PMID: 29058195
- PMCID: PMC5971093
- DOI: 10.1007/978-1-4939-7362-0_17
Stopped-Flow Fluorometric Ion Flux Assay for Ligand-Gated Ion Channel Studies
Abstract
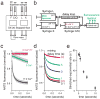
Quantitative investigations into functional properties of purified ion channel proteins using standard electrophysiological methods are challenging, in particular for the determination of average ion channel behavior following rapid changes in experimental conditions (e.g., ligand concentration). Here, we describe a method for determining the functional activity of liposome-reconstituted K+ channels using a stopped-flow fluorometric ion flux assay. Channel activity is quantified by measuring the rate of fluorescence decrease of a liposome-encapsulated fluorophore, specifically quenched by thallium ions entering the liposomes via open channels. This method is well suited for studying the lipid bilayer dependence of channel activity, the activation and desensitization kinetics of ligand-dependent K+ channels, and channel modulation by channel agonists, blockers, or other antagonists.
Keywords: ANTS quenching; Ion channel function; Liposomal ion flux assay; Stopped-flow assay; Thallium.
Figures

References
-
- Aidley DJ. The physiology of excitable cells. 4. Cambridge University Press; Cambridge, UK ; New York, NY, USA: 1998.
-
- Hille B. Ion channels of excitable membranes. 3. Sinauer; Sunderland, Mass: 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

