Efficacy of inhaled HYdrogen on neurological outcome following BRain Ischemia During post-cardiac arrest care (HYBRID II trial): study protocol for a randomized controlled trial
- PMID: 29058596
- PMCID: PMC5651618
- DOI: 10.1186/s13063-017-2246-3
Efficacy of inhaled HYdrogen on neurological outcome following BRain Ischemia During post-cardiac arrest care (HYBRID II trial): study protocol for a randomized controlled trial
Abstract
Background: Hydrogen gas inhalation (HI) improved survival and neurological outcomes in an animal model of post-cardiac arrest syndrome (PCAS). The feasibility and safety of HI for patients with PCAS was confirmed in a pilot study. The objective of this study is to evaluate the efficacy of HI for patients with PCAS.
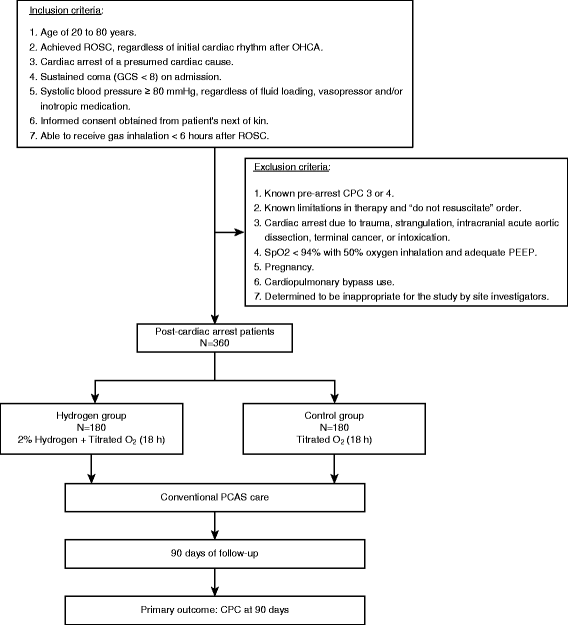
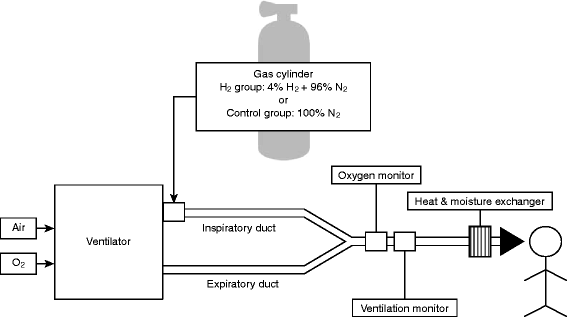
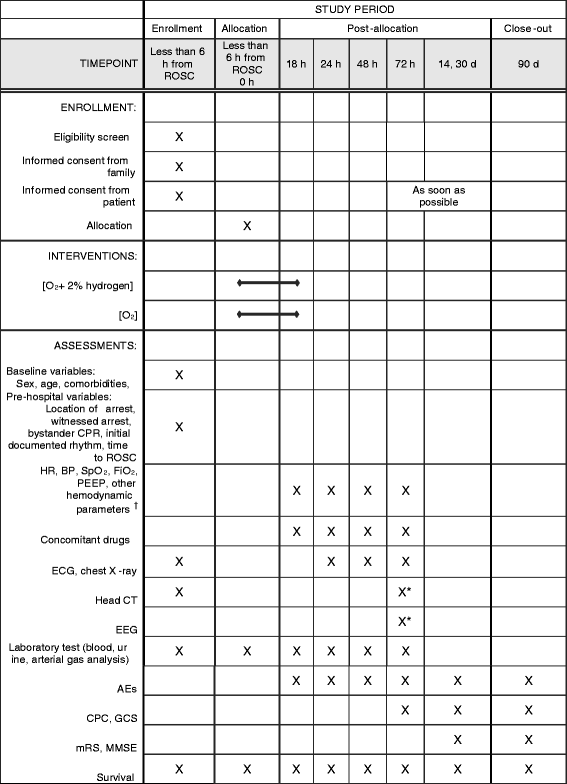
Methods/design: The efficacy of inhaled HYdrogen on neurological outcome following BRain Ischemia During post-cardiac arrest care (HYBRID II) trial is an investigator-initiated, randomized, double-blind, placebo-controlled trial designed to enroll 360 adult comatose (Glasgow Coma Scale score < 8) patients who will be resuscitated following an out-of-hospital cardiac arrest of a presumed cardiac cause. The patients will be randomized (1:1) to either the HI or control group. Patients in the HI group will inhale 2% hydrogen with 24% to 50% oxygen, and those in the control group will inhale 24% to 50% oxygen for 18 h after admission via mechanical ventilation. Multidisciplinary post-arrest care, including targeted temperature management (TTM) between 33 °C and 36 °C, will be provided in accordance with the latest guidelines. The primary outcome of interest is the 90-day neurological outcome, as evaluated using the Cerebral Performance Categories scale (CPC). The secondary outcomes of interest are the 90-day survival rate and other neurological outcomes. This study will provide 80% power to detect a 15% change in the proportion of patients with good neurological outcomes (CPCs of 1 and 2), from 50% to 65%, with an overall significance level of 0.05.
Discussion: The first multicenter randomized trial is underway to confirm the efficacy of HI on neurological outcomes in comatose out-of-hospital cardiac arrest survivors. Our study has the potential to address HI as an appealing and innovative therapeutic strategy for PCAS in combination with TTM.
Trials registration: University Hospital Medical Information Network (UMIN), 000019820 . Registered on 17 November 2015.
Keywords: Out-of-hospital cardiac arrest; hydrogen gas inhalation; post-cardiac arrest syndrome.
Conflict of interest statement
Ethics approval and consent to participate
This protocol was approved by the ethics committee of the Keio University School of Medicine (reference number 20150266). Additionally, this trial has been approved as an advanced medicine clinical trial by the Ministry of Health, Labor and Welfare of the Japanese government (reference number 1117-2). Ethics committee approval will be obtained at each participating hospital, and recruitment of patients will not start in those hospitals unless this study is approved by the ethics committee of that hospital. All amendments will be submitted for approval of the ethics committee of the Keio University School of Medicine and the Ministry of Health, Labor and Welfare of the Japanese government. A detailed description of obtaining consent to participate is provided within the text of this article.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–86. doi:10.1161/CIRCULATIONAHA.110.971002. Published errata appear in Circulation. 2011;123:e237 and Circulation. 2011;124:e403. - PubMed
-
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S465–82. doi: 10.1161/CIR.0000000000000262. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

