Endothelial jagged-2 sustains hematopoietic stem and progenitor reconstitution after myelosuppression
- PMID: 29058691
- PMCID: PMC5707154
- DOI: 10.1172/JCI92309
Endothelial jagged-2 sustains hematopoietic stem and progenitor reconstitution after myelosuppression
Abstract
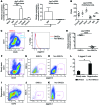
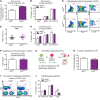
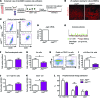
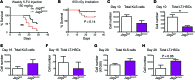
Angiocrine factors, such as Notch ligands, supplied by the specialized endothelial cells (ECs) within the bone marrow and splenic vascular niche play an essential role in modulating the physiology of adult hematopoietic stem and progenitor cells (HSPCs). However, the relative contribution of various Notch ligands, specifically jagged-2, to the homeostasis of HSPCs is unknown. Here, we show that under steady state, jagged-2 is differentially expressed in tissue-specific vascular beds, but its expression is induced in hematopoietic vascular niches after myelosuppressive injury. We used mice with EC-specific deletion of the gene encoding jagged-2 (Jag2) to demonstrate that while EC-derived jagged-2 was dispensable for maintaining the capacity of HSPCs to repopulate under steady-state conditions, by activating Notch2 it did contribute to the recovery of HSPCs in response to myelosuppressive conditions. Engraftment and/or expansion of HSPCs was dependent on the expression of endothelial-derived jagged-2 following myeloablation. Additionally, jagged-2 expressed in bone marrow ECs regulated HSPC cell cycle and quiescence during regeneration. Endothelial-deployed jagged-2 triggered Notch2/Hey1, while tempering Notch2/Hes1 signaling in HSPCs. Collectively, these data demonstrate that EC-derived jagged-2 activates Notch2 signaling in HSPCs to promote hematopoietic recovery and has potential as a therapeutic target to accelerate balanced hematopoietic reconstitution after myelosuppression.
Keywords: Adult stem cells; Bone marrow; Hematology; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

