Osteophytes and fracture calluses share developmental milestones and are diminished by unloading
- PMID: 29058776
- PMCID: PMC5877458
- DOI: 10.1002/jor.23779
Osteophytes and fracture calluses share developmental milestones and are diminished by unloading
Abstract
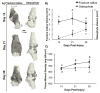
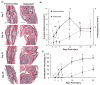
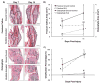
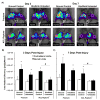
Osteophytes are a typical radiographic finding during osteoarthritis (OA), but the mechanisms leading to their formation are not well known. Comparatively, fracture calluses have been studied extensively; therefore, drawing comparisons between osteophytes and fracture calluses may lead to a deeper understanding of osteophyte formation. In this study, we compared the time courses of osteophyte and fracture callus formation, and investigated mechanisms contributing to development of these structure. Additionally, we investigated the effect of mechanical unloading on the formation of both fracture calluses and osteophytes. Mice underwent either transverse femoral fracture or non-invasive anterior cruciate ligament rupture. Fracture callus and osteophyte size and ossification were evaluated after 3, 5, 7, 14, 21, or 28 days. Additional mice were subjected to hindlimb unloading after injury for 3, 7, or 14 days. Protease activity and gene expression profiles after injury were evaluated after 3 or 7 days of normal ambulation or hindlimb unloading using in vivo fluorescence reflectance imaging (FRI) and quantitative PCR. We found that fracture callus and osteophyte growth achieved similar developmental milestones, but fracture calluses formed and ossified at earlier time points. Hindlimb unloading ultimately led to a threefold decrease in chondro/osteophyte area, and a twofold decrease in fracture callus area. Unloading was also associated with decreased inflammation and protease activity in injured limbs detected with FRI, particularly following ACL rupture. qPCR analysis revealed disparate cellular responses in fractured femurs and injured joints, suggesting that fracture calluses and osteophytes may form via different inflammatory, anabolic, and catabolic pathways. © 2017 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 36:699-710, 2018.
Keywords: fracture healing; hindlimb unloading; osteophytes; post-traumatic osteoarthritis.
© 2017 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

