RESIDUAL CHOROIDAL VESSELS IN ATROPHY CAN MASQUERADE AS CHOROIDAL NEOVASCULARIZATION ON OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY: Introducing a Clinical and Software Approach
- PMID: 29059100
- PMCID: PMC5910298
- DOI: 10.1097/IAE.0000000000001863
RESIDUAL CHOROIDAL VESSELS IN ATROPHY CAN MASQUERADE AS CHOROIDAL NEOVASCULARIZATION ON OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY: Introducing a Clinical and Software Approach
Abstract
Purpose: To present a postprocessing approach in optical coherence tomography angiography (OCTA) to facilitate the visualization and interpretation of lesions in age-related macular degeneration with coexisting atrophy and choroidal neovascularization (CNV).
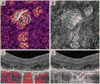
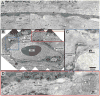
Methods: This retrospective study included 32 eyes of 26 patients with atrophy and treated CNV and 8 eyes with treatment-naive geographic atrophy. En face optical coherence tomography slabs highlighting atrophy were pseudocolored and merged with the corresponding OCTA. Cross-sectional optical coherence tomography and postprocessed OCTA were analyzed to identify CNV and normal choroidal vessels in relationship to the atrophy. We correlate the OCTA findings with those in a donor eye with treatment-naive geographic atrophy studied with transmission electronic microscopy.
Results: Medium-sized choroidal vessels were displaced anteriorly in areas of atrophy in all 40 eyes (100%), visualized in the choriocapillaris slab in all eyes, and in the outer retinal slab in 30 of 40 eyes (75.0%). Cross-sectional OCTA was used to confirm the presence of CNV. Postprocessing successfully highlighted the CNV and distinguished it from choroidal vessels in atrophy. Donor eye transmission electronic microscopy confirmed the anterior displacement of medium-sized choroidal vessels in geographic atrophy.
Conclusion: The anterior displacement of larger choroidal vessels in atrophy requires clinician vigilance to avoid misinterpreting these vessels as CNV on en face OCTA. Our proposed postprocessing approach offers a potential solution to facilitate the interpretation of en face OCTA in these cases. In the absence of other tools, clinicians are encouraged to rely on the location of flow relative to Bruch membrane on cross-sectional OCTA flow images.
Conflict of interest statement
Conflict of Interest: No conflicting relationship exists for any author.
Proprietary Interest: The authors have no proprietary interest in the subject of this manuscript.
Figures







Similar articles
-
Comparison of Automated versus Manually Modified OCT Angiography En Face Slabs for Detection of Choroidal Neovascularization.Ophthalmol Retina. 2020 May;4(5):471-480. doi: 10.1016/j.oret.2019.11.018. Epub 2019 Nov 30. Ophthalmol Retina. 2020. PMID: 32245653
-
CHOROIDAL BLOOD VESSELS IN RETINAL PIGMENT EPITHELIAL ATROPHY USING OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY.Retin Cases Brief Rep. 2019 Winter;13(1):88-93. doi: 10.1097/ICB.0000000000000542. Retin Cases Brief Rep. 2019. PMID: 28092315
-
DETECTION OF TREATMENT-NAIVE CHOROIDAL NEOVASCULARIZATION IN AGE-RELATED MACULAR DEGENERATION BY SWEPT SOURCE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY.Retina. 2018 Nov;38(11):2143-2149. doi: 10.1097/IAE.0000000000001832. Retina. 2018. PMID: 28902095
-
[A new approach for studying the retinal and choroidal circulation].Nippon Ganka Gakkai Zasshi. 2004 Dec;108(12):836-61; discussion 862. Nippon Ganka Gakkai Zasshi. 2004. PMID: 15656089 Review. Japanese.
-
Optical Coherence Tomography Angiography Features of Type 3 Neovascularization in Age-Related Macular Degeneration.Dev Ophthalmol. 2016;56:57-61. doi: 10.1159/000442779. Epub 2016 Mar 15. Dev Ophthalmol. 2016. PMID: 27023917 Review.
Cited by
-
Simultaneous GA and CNV/MNV: incidence, characteristics, and treatments.Graefes Arch Clin Exp Ophthalmol. 2025 May;263(5):1197-1212. doi: 10.1007/s00417-024-06721-5. Epub 2025 Apr 14. Graefes Arch Clin Exp Ophthalmol. 2025. PMID: 40229571 Free PMC article. Review.
-
Volume-Rendered Projection-Resolved OCT Angiography: 3D Lesion Complexity Is Associated With Therapy Response in Wet Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2018 Apr 1;59(5):1944-1952. doi: 10.1167/iovs.17-23361. Invest Ophthalmol Vis Sci. 2018. PMID: 29677356 Free PMC article.
-
Prevalence of Subclinical CNV and Choriocapillaris Nonperfusion in Fellow Eyes of Unilateral Exudative AMD on OCT Angiography.Transl Vis Sci Technol. 2018 Oct 1;7(5):19. doi: 10.1167/tvst.7.5.19. eCollection 2018 Sep. Transl Vis Sci Technol. 2018. PMID: 30280004 Free PMC article.
-
Detection of Reduced Retinal Vessel Density in Eyes with Geographic Atrophy Secondary to Age-Related Macular Degeneration Using Projection-Resolved Optical Coherence Tomography Angiography.Am J Ophthalmol. 2020 Jan;209:206-212. doi: 10.1016/j.ajo.2019.09.004. Epub 2019 Sep 14. Am J Ophthalmol. 2020. PMID: 31526797 Free PMC article.
-
Exploring the Relationship Between Multilayered Choroidal Neovascularization and Choriocapillaris Flow Deficits in AMD.Invest Ophthalmol Vis Sci. 2021 Mar 1;62(3):12. doi: 10.1167/iovs.62.3.12. Invest Ophthalmol Vis Sci. 2021. PMID: 33687474 Free PMC article.
References
-
- Lamoureux EL, Mitchell P, Rees G, et al. Impact of Early and Late Age-Related Macular Degeneration on Vision-Specific Functioning. Br J Ophthalmol. 2010 bjo. 2010.185207. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2006;355:1419–31. - PubMed
-
- Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal Bevacizumab (Avastin) for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2006;113:363–72. e5. - PubMed
-
- Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of Patients Losing Vision after 2 Years of Monthly Dosing in the Phase Iii Ranibizumab Clinical Trials. Ophthalmology. 2011;118:523–30. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

