Neurotensin receptor type 2 protects B-cell chronic lymphocytic leukemia cells from apoptosis
- PMID: 29059151
- PMCID: PMC5808079
- DOI: 10.1038/onc.2017.365
Neurotensin receptor type 2 protects B-cell chronic lymphocytic leukemia cells from apoptosis
Abstract
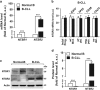
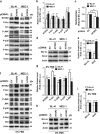
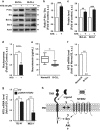
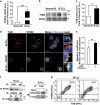
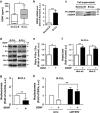
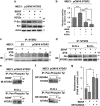
B-cell chronic lymphocytic leukemia (B-CLL) cells are resistant to apoptosis, and consequently accumulate to the detriment of normal B cells and patient immunity. Because current therapies fail to eradicate these apoptosis-resistant cells, it is essential to identify alternative survival pathways as novel targets for anticancer therapies. Overexpression of cell-surface G protein-coupled receptors drives cell transformation, and thus plays a critical role in malignancies. In this study, we identified neurotensin receptor 2 (NTSR2) as an essential driver of apoptosis resistance in B-CLL. NTSR2 was highly expressed in B-CLL cells, whereas expression of its natural ligand, neurotensin (NTS), was minimal in both B-CLL cells and patient plasma. Surprisingly, NTSR2 remained in a constitutively active phosphorylated state, caused not by a mutation-induced gain-of-function but rather by an interaction with the oncogenic tyrosine kinase receptor TrkB. Functional and biochemical characterization revealed that the NTSR2-TrkB interaction acts as a conditional oncogenic driver requiring the TrkB ligand brain-derived neurotrophic factor (BDNF), which unlike NTS is highly expressed in B-CLL cells. Together, NTSR2, TrkB and BDNF induce autocrine and/or paracrine survival pathways that are independent of mutation status and indolent or progressive disease course. The NTSR2-TrkB interaction activates survival signaling pathways, including the Src and AKT kinase pathways, as well as expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL. When NTSR2 was downregulated, TrkB failed to protect B-CLL cells from a drastic decrease in viability via typical apoptotic cell death, reflected by DNA fragmentation and Annexin V presentation. Together, our findings demonstrate that the NTSR2-TrkB interaction plays a crucial role in B-CLL cell survival, suggesting that inhibition of NTSR2 represents a promising targeted strategy for treating B-CLL malignancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kitada S, Pedersen IM, Schimmer AD, Reed JC. Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene 2002; 21: 3459–3474. - PubMed
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005; 352: 804–815. - PubMed
-
- Liu Y, An S, Ward R, Yang Y, Guo X-X, Li W et al. G protein-coupled receptors as promising cancer targets. Cancer Lett 2016; 376: 226–239. - PubMed
-
- Kehrl JH. Heterotrimeric G protein signaling: roles in immune function and fine-tuning by RGS proteins. Immunity 1998; 8: 1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

