Targeting mesothelin receptors with drug-loaded bacterial nanocells suppresses human mesothelioma tumour growth in mouse xenograft models
- PMID: 29059207
- PMCID: PMC5653298
- DOI: 10.1371/journal.pone.0186137
Targeting mesothelin receptors with drug-loaded bacterial nanocells suppresses human mesothelioma tumour growth in mouse xenograft models
Abstract
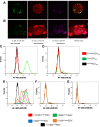
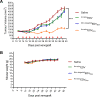
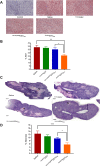
Human malignant mesothelioma is a chemoresistant tumour that develops from mesothelial cells, commonly associated with asbestos exposure. Malignant mesothelioma incidence rates in European countries are still rising and Australia has one of the highest burdens of malignant mesothelioma on a population basis in the world. Therapy using systemic delivery of free cytotoxic agents is associated with many undesirable side effects due to non-selectivity, and is thus dose-limited which limits its therapeutic potential. Therefore, increasing the selectivity of anti-cancer agents has the potential to dramatically enhance drug efficacy and reduce toxicity. EnGeneIC Dream Vectors (EDV) are antibody-targeted nanocells which can be loaded with cytotoxic drugs and delivered to specific cancer cells via bispecific antibodies (BsAbs) which target the EDV and a cancer cell-specific receptor, simultaneously. BsAbs were designed to target doxorubicin-loaded EDVs to cancer cells via cell surface mesothelin (MSLN). Flow cytometry was used to investigate cell binding and induction of apoptosis, and confocal microscopy to visualize internalization. Mouse xenograft models were used to assess anti-tumour effects in vivo, followed by immunohistochemistry for ex vivo evaluation of proliferation and necrosis. BsAb-targeted, doxorubicin-loaded EDVs were able to bind to and internalize within mesothelioma cells in vitro via MSLN receptors and induce apoptosis. In mice xenografts, the BsAb-targeted, doxorubicin-loaded EDVs suppressed the tumour growth and also decreased cell proliferation. Thus, the use of MSLN-specific antibodies to deliver encapsulated doxorubicin can provide a novel and alternative modality for treatment of mesothelioma.
Conflict of interest statement
Figures



Similar articles
-
Nanocell targeting using engineered bispecific antibodies.MAbs. 2015;7(1):53-65. doi: 10.4161/19420862.2014.985952. MAbs. 2015. PMID: 25523746 Free PMC article.
-
Microspheres targeted with a mesothelin antibody and loaded with doxorubicin reduce tumor volume of human mesotheliomas in xenografts.BMC Cancer. 2013 Sep 11;13:400. doi: 10.1186/1471-2407-13-400. BMC Cancer. 2013. PMID: 24024776 Free PMC article.
-
Targeted Doxorubicin-Loaded Bacterially Derived Nano-Cells for the Treatment of Neuroblastoma.Mol Cancer Ther. 2018 May;17(5):1012-1023. doi: 10.1158/1535-7163.MCT-17-0738. Epub 2018 Feb 28. Mol Cancer Ther. 2018. PMID: 29491149
-
Sialic acid-binding lectin from bullfrog eggs inhibits human malignant mesothelioma cell growth in vitro and in vivo.PLoS One. 2018 Jan 3;13(1):e0190653. doi: 10.1371/journal.pone.0190653. eCollection 2018. PLoS One. 2018. PMID: 29298350 Free PMC article.
-
Amatuximab and novel agents targeting mesothelin for solid tumors.Onco Targets Ther. 2017 Nov 8;10:5337-5353. doi: 10.2147/OTT.S145105. eCollection 2017. Onco Targets Ther. 2017. PMID: 29184420 Free PMC article. Review.
Cited by
-
Specific Targeting of Mesothelin-Expressing Malignant Cells Using Nanobody-Functionalized Magneto-Fluorescent Nanoassemblies.Int J Nanomedicine. 2024 Jan 20;19:633-650. doi: 10.2147/IJN.S435787. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38269255 Free PMC article.
-
Hitting the Bull's-Eye: Mesothelin's Role as a Biomarker and Therapeutic Target for Malignant Pleural Mesothelioma.Cancers (Basel). 2021 Aug 4;13(16):3932. doi: 10.3390/cancers13163932. Cancers (Basel). 2021. PMID: 34439085 Free PMC article. Review.
-
Nanotechnology-Employed Bacteria-Based Delivery Strategy for Enhanced Anticancer Therapy.Int J Nanomedicine. 2021 Dec 14;16:8069-8086. doi: 10.2147/IJN.S329855. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34934313 Free PMC article. Review.
-
Manipulating microRNAs for the Treatment of Malignant Pleural Mesothelioma: Past, Present and Future.Front Oncol. 2020 Feb 7;10:105. doi: 10.3389/fonc.2020.00105. eCollection 2020. Front Oncol. 2020. PMID: 32117755 Free PMC article. Review.
-
Silencing VDAC1 to Treat Mesothelioma Cancer: Tumor Reprograming and Altering Tumor Hallmarks.Biomolecules. 2022 Jun 27;12(7):895. doi: 10.3390/biom12070895. Biomolecules. 2022. PMID: 35883451 Free PMC article.
References
-
- Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103(4 Suppl):373S–6S. Epub 1993/04/01. . - PubMed
-
- Muers MF, Stephens RJ, Fisher P, Darlison L, Higgs CM, Lowry E, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet. 2008;371(9625):1685–94. doi: 10.1016/S0140-6736(08)60727-8 ; PubMed Central PMCID: PMC2431123. - DOI - PMC - PubMed
-
- Stumphius J, Meyer PB. Asbestos bodies and mesothelioma. The Annals of occupational hygiene. 1968;11(4):283–93. . - PubMed
-
- Carbone M, Bedrossian CW. The pathogenesis of mesothelioma. Seminars in diagnostic pathology. 2006;23(1):56–60. Epub 2006/10/19. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

