Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: A meta-analysis of peri-operative outcome
- PMID: 29059209
- PMCID: PMC5653345
- DOI: 10.1371/journal.pone.0186725
Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: A meta-analysis of peri-operative outcome
Expression of concern in
-
Expression of Concern: Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: A meta-analysis of peri-operative outcome.PLoS One. 2023 May 4;18(5):e0285545. doi: 10.1371/journal.pone.0285545. eCollection 2023. PLoS One. 2023. PMID: 37141199 Free PMC article. No abstract available.
Abstract
Objective: To assess whether neoadjuvant chemotherapy (NACT) is superior to primary debulking surgery (PDS) with regard to optimal cytoreduction, peri-operative morbidity, mortality, and quality of life (QOL) in advanced epithelial ovarian cancer (EOC).
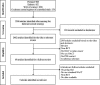
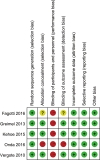
Methods: We searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, Web of Science, Registers of Clinical Trials for randomized controlled trials (RCTs) comparing NACT to PDS in women with Federation of International Gynaecologists and Obstetricians stage Ⅲ-Ⅳ EOC. RevMan 5.3 software was utilized for statistical analysis.
Results: Four RCTs involving 1,607 women with advanced EOC were included. Compared with PDS, NACT provided a higher rate of complete cytoreduction (risk ratio [RR], 1.95; 95% confidence interval [CI], 1.33 to 2.87), optimal cytoreduction (RR: 1.61 [95%CI: 1.05 to 2.47]), but there was no significant difference in residual disease 0-1 cm (p = 0.49). NACT was associated with lower peri-operative morbidity with respect to infection (RR: 0.30 [95% CI: 0.16 to 0.56]), gastrointestinal fistula (RR: 0.24 [95% CI: 0.06 to 0.95]), any grade 3 or 4 adverse event (RR: 0.29 [95% CI: 0.11 to 0.78]), and less post-surgical death within 28 days (RR: 0.14 [95% CI: 0.04 to 0.49]). NACT provided better QOL in terms of fatigue (weight mean difference [WMD], -3.28; [95% CI: -3.99 to -2.57]), role functioning (WMD: 5.29 [95% CI: 4.44 to 6.14]), emotional functioning (WMD: 6.19 [95% CI: 5.57 to 6.82]), and cognitive functioning (WMD: 1.02 [95% CI: 0.43 to 1.61]) at 6-month follow-up compared with PDS.
Conclusions: NACT is associated with superior optimal cytoreduction, lower peri-operative morbidity as well as post-surgical mortality, and better QOL compared to initial surgery in patients with advanced EOC. Future research should focus on improving the efficacy of NACT.
Conflict of interest statement
Figures




References
-
- Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D, et al. Cancer statistics for Hispanics/Latinos, 2015. CA: a cancer journal for clinicians. 2015;65: 457–80. doi: 10.3322/caac.21314 - DOI - PubMed
-
- Minig L, Zorrero C, Iserte PP, Poveda A. Selecting the best strategy of treatment in newly diagnosed advanced-stage ovarian cancer patients. World journal of methodology. 2015;5: 196–202. doi: 10.5662/wjm.v5.i4.196 - DOI - PMC - PubMed
-
- Seidman JD, Yemelyanova A, Cosin JA, Smith A, Kurman RJ. Survival rates for international federation of gynecology and obstetrics stage III ovarian carcinoma by cell type: a study of 262 unselected patients with uniform pathologic review. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2012;22: 367–71. doi: 10.1097/IGC.0b013e31823c6f80 - DOI - PubMed
-
- Fader AN, Rose PG. Role of surgery in ovarian carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25: 2873–83. doi: 10.1200/jco.2007.11.0932 - DOI - PubMed
-
- Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2011;21: 750–5. doi: 10.1097/IGC.0b013e31821b2568 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

