Protective effect of dexamethasone on 5-FU-induced oral mucositis in hamsters
- PMID: 29059216
- PMCID: PMC5653368
- DOI: 10.1371/journal.pone.0186511
Protective effect of dexamethasone on 5-FU-induced oral mucositis in hamsters
Abstract
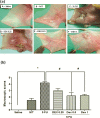
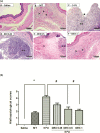
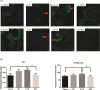
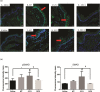
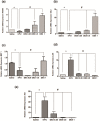
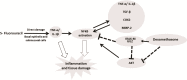
Oral mucositis (OM) is an important side effect of cancer treatment, characterized by ulcerative lesions in the mucosa of patients undergoing radiotherapy or chemotherapy, which has marked effects on patient quality of life and cancer therapy continuity. Considering that few protocols have demonstrated efficacy in preventing this side effect, the aim of this study was to examine the effect of dexamethasone (DEX) on OM induced by 5-fluorouracil (5-FU) in hamsters by studying signaling pathways. OM was induced in hamsters by 5-FU followed by mechanical trauma (MT) on day 4. On day 10, the animals were euthanized. The experimental groups included saline, MT, 5-FU, and DEX (0.25, 0.5, or 1 mg/kg). Macroscopic, histopathological, and immunohistochemical analyses as well as immunofluorescence experiments were performed on the oral mucosa of the animals. The oral mucosal samples were analyzed by enzyme-linked immunosorbent assays, and quantitative real-time polymerase chain reaction (qPCR). DEX (0.5 or 1 mg/kg) reduced inflammation and ulceration of the oral mucosa of hamsters. In addition, DEX (1 mg/kg) reduced the cytokine levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and macrophage migration inhibitory factor (MIF). DEX (1 mg/kg) also reduced the immunoexpression of cyclooxygenase (COX)-2, matrix metalloproteinase (MMP)-2, transforming growth factor (TGF)-β, MIF, Smad 2/3, Smad 2/3 phosphorylated and NFκB p65 in the jugal mucosa. Finally, DEX (1 mg/kg) increased interleukin-1 receptor-associated kinase 3 (IRAK-M), glucocorticoid-induced leucine zipper (GILZ), and mitogen-activated protein kinase (MKP1) gene expression and reduced NFκB p65 and serine threonine kinase (AKt) gene expression, relative to the 5-FU group. Thus, DEX improved OM induced by 5-FU in hamsters.
Conflict of interest statement
Figures


References
-
- Silverman S Jr. (2007) Diagnosis and management of oral mucositis. J Support Oncol 5: 13–21. - PubMed
-
- Lalla RV, Sonis ST, Peterson DE (2008) Management of oral mucositis in patients who have cancer. Dent Clin North Am 52: 61–77, viii. doi: 10.1016/j.cden.2007.10.002 - DOI - PMC - PubMed
-
- Sonis ST (2010) New thoughts on the initiation of mucositis. Oral Dis 16: 597–600. doi: 10.1111/j.1601-0825.2010.01681.x - DOI - PubMed
-
- Sonis ST (2007) Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol 5: 3–11. - PubMed
-
- Logan RM, Gibson RJ, Sonis ST, Keefe DM (2007) Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol 43: 395–401. doi: 10.1016/j.oraloncology.2006.04.011 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

