Clock-dependent and system-driven oscillators interact in the suprachiasmatic nuclei to pace mammalian circadian rhythms
- PMID: 29059248
- PMCID: PMC5653358
- DOI: 10.1371/journal.pone.0187001
Clock-dependent and system-driven oscillators interact in the suprachiasmatic nuclei to pace mammalian circadian rhythms
Abstract
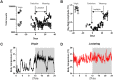
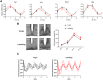
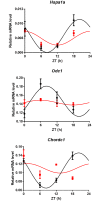
Circadian clocks drive biological rhythms with a period of approximately 24 hours and keep in time with the outside world through daily resetting by environmental cues. While this external entrainment has been extensively investigated in the suprachiasmatic nuclei (SCN), the role of internal systemic rhythms, including daily fluctuations in core temperature or circulating hormones remains debated. Here, we show that lactating mice, which exhibit dampened systemic rhythms, possess normal molecular clockwork but impaired rhythms in both heat shock response gene expression and electrophysiological output in their SCN. This suggests that body rhythms regulate SCN activity downstream of the clock. Mathematical modeling predicts that systemic feedback upon the SCN functions as an internal oscillator that accounts for in vivo and ex vivo observations. Thus we are able to propose a new bottom-up hierarchical organization of circadian timekeeping in mammals, based on the interaction in the SCN between clock-dependent and system-driven oscillators.
Conflict of interest statement
Figures



Similar articles
-
Circadian PER2::LUC rhythms in the olfactory bulb of freely moving mice depend on the suprachiasmatic nucleus but not on behaviour rhythms.Eur J Neurosci. 2015 Dec;42(12):3128-37. doi: 10.1111/ejn.13111. Eur J Neurosci. 2015. PMID: 26489367
-
Entrainment of circadian clocks in mammals by arousal and food.Essays Biochem. 2011 Jun 30;49(1):119-36. doi: 10.1042/bse0490119. Essays Biochem. 2011. PMID: 21819388
-
Advanced light-entrained activity onsets and restored free-running suprachiasmatic nucleus circadian rhythms in per2/dec mutant mice.Chronobiol Int. 2011 Nov;28(9):737-50. doi: 10.3109/07420528.2011.607374. Chronobiol Int. 2011. PMID: 22080784
-
Mammalian circadian signaling networks and therapeutic targets.Nat Chem Biol. 2007 Oct;3(10):630-9. doi: 10.1038/nchembio.2007.37. Nat Chem Biol. 2007. PMID: 17876320 Review.
-
Regulation and function of extra-SCN circadian oscillators in the brain.Acta Physiol (Oxf). 2020 May;229(1):e13446. doi: 10.1111/apha.13446. Epub 2020 Feb 6. Acta Physiol (Oxf). 2020. PMID: 31965726 Review.
Cited by
-
A minimal model of peripheral clocks reveals differential circadian re-entrainment in aging.Chaos. 2023 Sep 1;33(9):093104. doi: 10.1063/5.0157524. Chaos. 2023. PMID: 37669108 Free PMC article.
-
Pregnancy rest-activity patterns are related to salivary cortisol rhythms and maternal-fetal health indicators in women from a disadvantaged population.PLoS One. 2020 Mar 3;15(3):e0229567. doi: 10.1371/journal.pone.0229567. eCollection 2020. PLoS One. 2020. PMID: 32126104 Free PMC article.
-
Molecular and Cellular Networks in The Suprachiasmatic Nuclei.Int J Mol Sci. 2019 Apr 25;20(8):2052. doi: 10.3390/ijms20082052. Int J Mol Sci. 2019. PMID: 31027315 Free PMC article. Review.
-
STING orchestrates the crosstalk between polyunsaturated fatty acid metabolism and inflammatory responses.Cell Metab. 2022 Jan 4;34(1):125-139.e8. doi: 10.1016/j.cmet.2021.12.007. Cell Metab. 2022. PMID: 34986331 Free PMC article.
-
A Journey in the Brain's Clock: In Vivo Veritas?Biology (Basel). 2023 Aug 15;12(8):1136. doi: 10.3390/biology12081136. Biology (Basel). 2023. PMID: 37627020 Free PMC article. Review.
References
-
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–46. Epub 2004/02/14. doi: 10.1073/pnas.0308709101 . - DOI - PMC - PubMed
-
- Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst GT, et al. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol. 2002;12(13):1130–3. Epub 2002/07/18. doi: 10.1016/S0960-9822(02)00923-5 . - DOI - PubMed
-
- Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci. 2002;5(5):399–400. Epub 2002/04/16. doi: 10.1038/nn843 . - DOI - PubMed
-
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12(10):553–69. doi: 10.1038/nrn3086 . - DOI - PMC - PubMed
-
- Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron. 2017;93(6):1420–35 e5. Epub 2017/03/14. doi: 10.1016/j.neuron.2017.02.030 . - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

