A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): A randomized, controlled non-inferiority trial
- PMID: 29059253
- PMCID: PMC5653205
- DOI: 10.1371/journal.pmed.1002411
A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): A randomized, controlled non-inferiority trial
Abstract
Background: The management of childhood infections remains inadequate in resource-limited countries, resulting in high mortality and irrational use of antimicrobials. Current disease management tools, such as the Integrated Management of Childhood Illness (IMCI) algorithm, rely solely on clinical signs and have not made use of available point-of-care tests (POCTs) that can help to identify children with severe infections and children in need of antibiotic treatment. e-POCT is a novel electronic algorithm based on current evidence; it guides clinicians through the entire consultation and recommends treatment based on a few clinical signs and POCT results, some performed in all patients (malaria rapid diagnostic test, hemoglobin, oximeter) and others in selected subgroups only (C-reactive protein, procalcitonin, glucometer). The objective of this trial was to determine whether the clinical outcome of febrile children managed by the e-POCT tool was non-inferior to that of febrile children managed by a validated electronic algorithm derived from IMCI (ALMANACH), while reducing the proportion with antibiotic prescription.
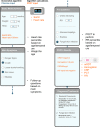
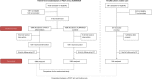
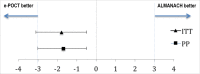
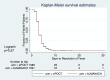
Methods and findings: We performed a randomized (at patient level, blocks of 4), controlled non-inferiority study among children aged 2-59 months presenting with acute febrile illness to 9 outpatient clinics in Dar es Salaam, Tanzania. In parallel, routine care was documented in 2 health centers. The primary outcome was the proportion of clinical failures (development of severe symptoms, clinical pneumonia on/after day 3, or persistent symptoms at day 7) by day 7 of follow-up. Non-inferiority would be declared if the proportion of clinical failures with e-POCT was no worse than the proportion of clinical failures with ALMANACH, within statistical variability, by a margin of 3%. The secondary outcomes included the proportion with antibiotics prescribed on day 0, primary referrals, and severe adverse events by day 30 (secondary hospitalizations and deaths). We enrolled 3,192 patients between December 2014 and February 2016 into the randomized study; 3,169 patients (e-POCT: 1,586; control [ALMANACH]: 1,583) completed the intervention and day 7 follow-up. Using e-POCT, in the per-protocol population, the absolute proportion of clinical failures was 2.3% (37/1,586), as compared with 4.1% (65/1,583) in the ALMANACH arm (risk difference of clinical failure -1.7, 95% CI -3.0, -0.5), meeting the prespecified criterion for non-inferiority. In a non-prespecified superiority analysis, we observed a 43% reduction in the relative risk of clinical failure when using e-POCT compared to ALMANACH (risk ratio [RR] 0.57, 95% CI 0.38, 0.85, p = 0.005). The proportion of severe adverse events was 0.6% in the e-POCT arm compared with 1.5% in the ALMANACH arm (RR 0.42, 95% CI 0.20, 0.87, p = 0.02). The proportion of antibiotic prescriptions was substantially lower, 11.5% compared to 29.7% (RR 0.39, 95% CI 0.33, 0.45, p < 0.001). Using e-POCT, the most common indication for antibiotic prescription was severe disease (57%, 103/182 prescriptions), while it was non-severe respiratory infections using the control algorithm (ALMANACH) (70%, 330/470 prescriptions). The proportion of clinical failures among the 544 children in the routine care cohort was 4.6% (25/544); 94.9% (516/544) of patients received antibiotics on day 0, and 1.1% (6/544) experienced severe adverse events. e-POCT achieved a 49% reduction in the relative risk of clinical failure compared to routine care (RR 0.51, 95% CI 0.31, 0.84, p = 0.007) and lowered antibiotic prescriptions to 11.5% from 94.9% (p < 0.001). Though this safety study was an important first step to evaluate e-POCT, its true utility should be evaluated through future implementation studies since adherence to the algorithm will be an important factor in making use of e-POCT's advantages in terms of clinical outcome and antibiotic prescription.
Conclusions: e-POCT, an innovative electronic algorithm using host biomarker POCTs, including C-reactive protein and procalcitonin, has the potential to improve the clinical outcome of children with febrile illnesses while reducing antibiotic use through improved identification of children with severe infections, and better targeting of children in need of antibiotic prescription.
Trial registration: ClinicalTrials.gov NCT02225769.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Burton DC, Flannery B, Onyango B, Larson C, Alaii J, Zhang X, et al. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Heal Popul Nutr. 2011;29:61–70. doi: 10.3329/jhpn.v29i1.7567 - DOI - PMC - PubMed
-
- Shao AF, Rambaud-Althaus C, Samaka J, Faustine AF, Perri-Moore S, Swai N, et al. New algorithm for managing childhood illness using mobile technology (ALMANACH): a controlled non-inferiority study on clinical outcome and antibiotic use in Tanzania. PLoS ONE. 2015;10:e0132316 doi: 10.1371/journal.pone.0132316 - DOI - PMC - PubMed
-
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2014;385:430–40. doi: 10.1016/S0140-6736(14)61698-6 - DOI - PubMed
-
- Risk R, Naismith H, Burnett A, Moore SE, Cham M, Unger S. Rational prescribing in paediatrics in a resource-limited setting. Arch Dis Child. 2013;98:503–9. doi: 10.1136/archdischild-2012-302987 - DOI - PubMed
-
- Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–93. doi: 10.1016/S1473-3099(05)70189-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

