Effect of Financial Incentives on Glucose Monitoring Adherence and Glycemic Control Among Adolescents and Young Adults With Type 1 Diabetes: A Randomized Clinical Trial
- PMID: 29059263
- PMCID: PMC6583649
- DOI: 10.1001/jamapediatrics.2017.3233
Effect of Financial Incentives on Glucose Monitoring Adherence and Glycemic Control Among Adolescents and Young Adults With Type 1 Diabetes: A Randomized Clinical Trial
Abstract
Importance: Glycemic control often deteriorates during adolescence and the transition to young adulthood for patients with type 1 diabetes. The inability to manage type 1 diabetes effectively during these years is associated with poor glycemic control and complications from diabetes in adult life.
Objective: To determine the effect of daily financial incentives on glucose monitoring adherence and glycemic control in adolescents and young adults with type 1 diabetes.
Design, setting, and participants: The Behavioral Economic Incentives to Improve Glycemic Control Among Adolescents and Young Adults With Type 1 Diabetes (BE IN CONTROL) study was an investigator-blinded, 6-month, 2-arm randomized clinical trial conducted between January 22 and November 2, 2016, with 3-month intervention and follow-up periods. Ninety participants (aged 14-20) with suboptimally controlled type 1 diabetes (hemoglobin A1c [HbA1c] >8.0%) were recruited from the Diabetes Center for Children at the Children's Hospital of Philadelphia.
Interventions: All participants were given daily blood glucose monitoring goals of 4 or more checks per day with 1 or more level within the goal range (70-180 mg/dL) collected with a wireless glucometer. The 3-month intervention consisted of a $60 monthly incentive in a virtual account, from which $2 was subtracted for every day of nonadherence to the monitoring goals. During a 3-month follow-up period, the intervention was discontinued.
Main outcomes and measures: The primary outcome was change in HbA1c levels at 3 months. Secondary outcomes included adherence to glucose monitoring and change in HbA1c levels at 6 months. All analyses were by intention to treat.
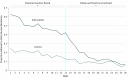
Results: Of the 181 participants screened, 90 (52 [57.8%] girls) were randomized to the intervention (n = 45) or control (n = 45) arms. The mean (SD) age was 16.3 (1.9) years. The intervention group had significantly greater adherence to glucose monitoring goals in the incentive period (50.0% vs 18.9%; adjusted difference, 27.2%; 95% CI, 9.5% to 45.0%; P = .003) but not in the follow-up period (15.3% vs 8.7%; adjusted difference, 3.9%; 95% CI, -2.0% to 9.9%; P = .20). The change in HbA1c levels from baseline did not differ significantly between groups at 3 months (adjusted difference, -0.08%; 95% CI, -0.69% to 0.54%; P = .80) or 6 months (adjusted difference, 0.03%; 95% CI, -0.55% to 0.60%; P = .93).
Conclusions and relevance: Among adolescents and young adults with type 1 diabetes, daily financial incentives improved glucose monitoring adherence during the incentive period but did not significantly improve glycemic control.
Trial registration: clinicaltrials.gov Identifier: NCT02568501.
Conflict of interest statement
Figures
Comment in
-
Motivating Health Behaviors in Adolescents Through Behavioral Economics.JAMA Pediatr. 2017 Dec 1;171(12):1145-1146. doi: 10.1001/jamapediatrics.2017.3464. JAMA Pediatr. 2017. PMID: 29059260 Free PMC article. No abstract available.
References
-
- Miller KM, Foster NC, Beck RW, et al. ; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange Clinic registry. Diabetes Care. 2015;38(6):971-978. - PubMed
-
- Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171-e1179. - PubMed
-
- Standards of medical care in diabetes–2015: summary of revisions. Diabetes Care. 2015;38(suppl):S4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

