Cracking the Betel Nut: Cholinergic Activity of Areca Alkaloids and Related Compounds
- PMID: 29059390
- PMCID: PMC6528145
- DOI: 10.1093/ntr/ntx187
Cracking the Betel Nut: Cholinergic Activity of Areca Alkaloids and Related Compounds
Abstract
Introduction: The use of betel quid is the most understudied major addiction in the world. The neuropsychological activity of betel quid has been attributed to alkaloids of Areca catechu. With the goal of developing novel addiction treatments, we evaluate the muscarinic and nicotinic activity of the four major Areca alkaloids: arecoline, arecaidine, guvacoline, and guvacine and four structurally related compounds.
Methods: Acetylcholine receptors were expressed in Xenopus oocytes and studied with two-electrode voltage clamp.
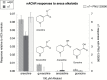
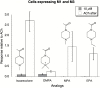
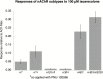
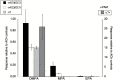
Results: Both arecoline- and guvacoline-activated muscarinic acetylcholine receptors (mAChR), while only arecoline produced significant activation of nicotinic AChR (nAChR). We characterized four additional arecoline-related compounds, seeking an analog that would retain selective activity for a α4* nAChR, with diminished effects on mAChR and not be a desensitizer of α7 nAChR. We show that this profile is largely met by isoarecolone. Three additional arecoline analogs were characterized. While the quaternary dimethyl analog had a broad range of activities, including activation of mAChR and muscle-type nAChR, the methyl analog only activated a range of α4* nAChR, albeit with low potency. The ethyl analog had no detectable cholinergic activity.
Conclusions: Evidence indicates that α4* nAChR are at the root of nicotine addiction, and this may also be the case for betel addiction. Our characterization of isoarecolone and 1-(4-methylpiperazin-1-yl) ethanone as truly selective α4*nAChR selective partial agonists with low muscarinic activity may point toward a promising new direction for the development of drugs to treat both nicotine and betel addiction.
Implications: Nearly 600 million people use Areca nut, often with tobacco. Two of the Areca alkaloids are muscarinic acetylcholine receptor agonists, and one, arecoline, is a partial agonist for the α4* nicotinic acetylcholine receptors (nAChR) associated with tobacco addiction. The profile of arecoline activity suggested its potential to be used as a scaffold for developing new tobacco cessation drugs if analogs can be identified that retain the same nicotinic receptor selectivity without muscarinic activity. We report that isoarecolone is a selective partial agonist for α4* nAChR with minimal muscarinic activity and 1-(4-methylpiperazin-1-yl) ethanone has similar nAChR selectivity and no detectable muscarinic action.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Little MA, Papke RL. Betel, the orphan addiction. J Addict Res Ther. 2015;6(3):130–132.
-
- Lord GA, Lim CK, Warnakulasuriya S, Peters TJ. Chemical and analytical aspects of areca nut. Addict Biol. 2002;7(1):99–102. - PubMed
-
- Lee CH, Chiang SL, Ko AM, et al. . Betel-quid dependence domains and syndrome associated with betel-quid ingredients among chewers: an Asian multi-country evidence. Addiction. 2014;109(7):1194–1204. - PubMed
-
- Winstock AR, Trivedy CR, Warnakulasuriya KA, Peters TJ. A dependency syndrome related to areca nut use: some medical and psychological aspects among areca nut users in the Gujarat community in the UK. Addict Biol. 2000;5(2):173–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

