Facilitated Extinction Training to Improve Pharmacotherapy for Smoking Cessation: A Pilot Feasibility Trial
- PMID: 29059409
- PMCID: PMC7868958
- DOI: 10.1093/ntr/ntx203
Facilitated Extinction Training to Improve Pharmacotherapy for Smoking Cessation: A Pilot Feasibility Trial
Abstract
Introduction: Varenicline reduces smoking satisfaction during the pre-cessation run-in period, which may contribute to extinction of cravings and smoking behavior. Research indicates that efficacy is enhanced when the run-in period is increased from 1 to 4 weeks, providing a longer extinction opportunity. We hypothesized that efficacy could be further enhanced by harnessing basic and applied research on extinction. We developed a pre-cessation extinction-facilitating intervention and tested its feasibility in a pilot trial.
Methods: The facilitated extinction (FE) intervention comprised brief counseling and workbook-recommending strategies to maximize extinction processes during the run-in, including instructions to smoke at a normal rate across contexts and cues, and use of an extinction cue to enhance generalization. Participants were randomly assigned to one of three varenicline interventions: standard (1-week run-in), extended (4-week run-in), and extended + FE. Interventions were delivered prior to the target quit date (TQD). Assessments were conducted in weeks 1 and 4 pre-TQD and 1 and 3 months post-TQD, with focus on feasibility indices.
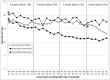
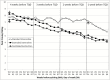
Results: Recruitment and retention goals were met (N = 58). Treatment satisfaction was high across groups. The majority of FE participants adhered to instructions and maintained their usual smoking rate during the run-in period. Greater decreases in craving and smoking satisfaction were observed among participants in both extended groups versus the standard group (p < .005).
Conclusions: Feasibility was demonstrated. Participants adhered to the FE intervention, thereby optimizing the number and variety of extinction trials. Findings support testing the novel FE smoking cessation intervention in a fully powered trial.
Implications: This study expands the research on the clinical benefits of extending the pre-cessation run-in period of varenicline. It introduces the hypothesis that further benefit might be achieved by translating basic behavioral research, as well as cue-exposure research and therapy for other disorders, to improve the extinction and generalization processes thought to underlie much of varenicline's effect. A FE intervention was developed and found acceptable to smokers and feasible to implement in a research setting. The study sets the stage for a subsequent randomized controlled trial.
Figures


References
-
- Gonzales D, Rennard SI, Nides Met al. ; Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. - PubMed
-
- Jorenby DE, Hays JT, Rigotti NAet al. ; Varenicline Phase 3 Study Group Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. - PubMed
-
- Nides M, Oncken C, Gonzales Det al. . Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568. - PubMed
-
- Oncken C, Gonzales D, Nides Met al. . Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166(15):1571–1577. - PubMed
-
- Ray LA, Lunny K, Bujarski S, Moallem N, Krull JL, Miotto K. The effects of varenicline on stress-induced and cue-induced craving for cigarettes. Drug Alcohol Depend. 2013;131(1-2):136–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

