Development of a core outcome set for clinical trials in basal cell carcinoma: study protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey
- PMID: 29061190
- PMCID: PMC5654122
- DOI: 10.1186/s13063-017-2244-5
Development of a core outcome set for clinical trials in basal cell carcinoma: study protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey
Abstract
Background: Basal cell carcinoma is the most common skin cancer worldwide. Treatment options include both surgical and topical modalities. Although risk of metastasis is low, basal cell carcinoma can be invasive and infiltrate important underlying structures such as bone or cartilage. While many clinical trials examining therapies for basal cell carcinoma exist, the lack of consensus in outcome reporting across all trials poses a concern. Proper evaluation and comparison of treatment modalities is challenging. In order to address the inconsistencies present, this project aims to determine a core set of outcomes which should be evaluated in all clinical trials of basal cell carcinoma.
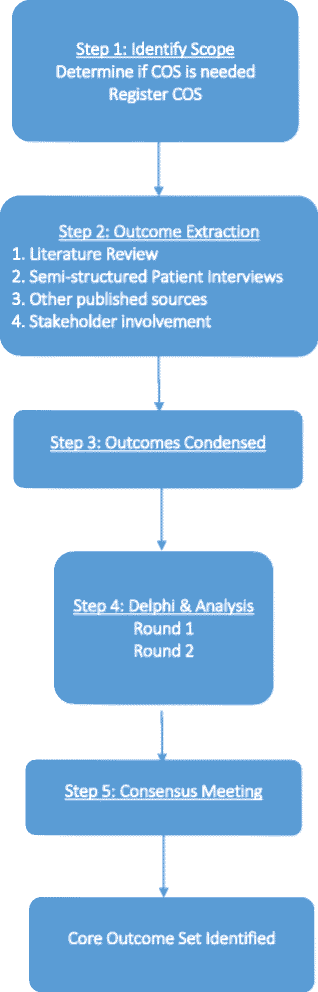
Methods/design: Outcomes will be extracted over four phases: (1) a systematic literature review, (2) patient interviews, (3) other published sources, and (4) stakeholder involvement. Potential outcomes will then be examined by the Steering Committee, who may add or remove outcomes. The Delphi process will then be performed to condense the list of outcomes generated. Two rounds of Delphi surveys will be performed with two groups of participants - physicians and patients. A consensus meeting with relevant stakeholders will be conducted after the Delphi exercise to further select outcomes, taking into account participant scores. By the end of the meeting, members will vote and decide on a final recommended set of core outcomes. For the duration of the study, we will be in collaboration with both the Core Outcome Measures in Effectiveness Trials (COMET) initiative and the Cochrane Skin Group - Core Outcome Set Initiative (CSG-COUSIN).
Discussion: This study aims to develop a core outcome set to guide assessment in clinical trials on basal cell carcinoma. The end-goal is to improve the consistency of outcome reporting and allow proper evaluation of treatment effectiveness.
Keywords: Basal cell carcinoma; Consensus; Core outcome set; Delphi; Stakeholders; Systematic review.
Conflict of interest statement
Ethics approval and consent to participate
A systematic review of the academic literature will be performed with the help of data extractors across four institutions. Ethical approval was not applicable for the review of the academic literature.
Ethical approval and consent to participate for the interviews and Delphi study has been granted from the Northwestern University Institutional Review Board (IRB) protocol ID: STU00201637. Informed consent will be obtained from each participant.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Miller SJ, Alam M, Andersen J, Berg D, Bichakjian CK, Bowen G, Cheney RT, Glass LF, Grekin RC, Kessinger A, Lee NY, Liegeois N, Lydiatt DD, Michalski J, Morrison WH, Nehal KS, Nelson KC, Nghiem P, Olencki T, Perlis CS, Rosenberg EW, Shaha AR, Urist MM, Wang LC, Zic JA. Basal cell and squamous cell skin cancers. J Natl Compr Canc Netw. 2010;8(8):836–64. doi: 10.6004/jnccn.2010.0062. - DOI - PubMed
-
- van Dam RM, Huang Z, Rimm EB, Weinstock MA, Spiegelman D, Colditz GA, Willett WC, Giovannucci E. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150(5):459–68. doi: 10.1093/oxfordjournals.aje.a010034. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical