Pharmacodynamic Monitoring Predicts Outcomes of CCR5 Blockade as Graft-versus-Host Disease Prophylaxis
- PMID: 29061535
- PMCID: PMC5826857
- DOI: 10.1016/j.bbmt.2017.10.028
Pharmacodynamic Monitoring Predicts Outcomes of CCR5 Blockade as Graft-versus-Host Disease Prophylaxis
Abstract
Blocking lymphocyte trafficking after allogeneic hematopoietic stem cell transplantation is a promising strategy to prevent graft-versus-host disease (GVHD) while preserving the graft-versus-tumor response. Maraviroc, a CCR5 antagonist, has shown promise in clinical trials, presumably by disrupting the migration of effector cells to GVHD target organs. We describe a phosphoflow assay to quantify CCR5 blockade during treatment with maraviroc and used it to evaluate 28 patients in a phase II study. We found that insufficient blockade of CCR5 was associated with significantly worse overall survival (HR, 10.6; 95% CI, 2.2 to 52.0; P = .004) and higher rates of nonrelapse mortality (HR, 146; 95% CI, 1.0 to 20,600; P = .04) and severe acute GVHD (HR, 12; 95% CI, 1.9 to 76.6; P = .009). In addition, we found that pretransplant high surface expression of CCR5 on recipient T cells predicted higher nonrelapse mortality and worse GVHD- and relapse-free survival. Our results demonstrate that pharmacodynamic monitoring of CCR5 blockade unravels interpatient variability in the response to therapy and may serve as a clinically informative biomarker.
Keywords: Chemokine receptor blockade; GVHD; Maraviroc.
Copyright © 2017 The American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Figures


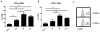

References
-
- Johnston L. Acute graft-versus-host disease: differing risk with differing graft sources and conditioning intensity. Best Pract Res Clin Haematol. 2008;21:177–192. - PubMed
-
- Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, Hamada H, Asakura H, Ishikawa H, Matsushima K. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4:154–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

