Assessing Noninferiority in Treatment Trials for Severe Infectious Diseases: an Extension to the Entire Follow-Up Period Using a Cure-Death Multistate Model
- PMID: 29061757
- PMCID: PMC5740315
- DOI: 10.1128/AAC.01691-17
Assessing Noninferiority in Treatment Trials for Severe Infectious Diseases: an Extension to the Entire Follow-Up Period Using a Cure-Death Multistate Model
Abstract
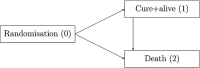
In current and former clinical trials for the development of antibacterial drugs, various primary endpoints have been used, and treatment effects are evaluated mostly in noninferiority analyses at the end of follow-up, which varies between studies. A more convincing and highly patient-relevant statement would be a noninferiority assessment over the entire follow-up period with cure and death as coprimary endpoints, while preserving the desired alpha level for statistical testing. To account for the time-dynamic pattern of cure and death, we apply a cure-death multistate model. The endpoint of interest is "get cured and stay alive over time." Noninferiority between treatments over the entire follow-up period is studied by means of one-sided confidence bands provided by a flexible resampling technique. We illustrate the technique by applying it to a recently published study and establish noninferiority in being cured and alive over a time frame of interest for the entire population, patients with hospital-acquired pneumonia, but not for the subset of patients with ventilator-associated pneumonia. Our analysis improves the original results in the sense that our endpoint is more patient benefiting, a stronger noninferiority statement is demonstrated, and the time dependency of cure and death, competing events, and different follow-up times is captured. Multistate methodology combined with confidence bands adds a valuable statistical tool for clinical trials in the context of infection control. The framework is not restricted to the cure-death model but can be adapted to more complex multistate endpoints and equivalence or superiority analyses.
Keywords: endpoints; hospital-acquired infection; multistate model; noninferiority.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Timsit JF, de Kraker MEA, Sommer H, Weiss E, Bettiol E, Wolkewitz M, Wilson D, Harbarth S. 2017. Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: a white paper from the COMBACTE network. Intensive Care Med 43:1002–1012. doi:10.1007/s00134-017-4802-4. - DOI - PMC - PubMed
-
- European Medicines Agency. 2013. Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... Accessed 15 August 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

