The origin of heredity in protocells
- PMID: 29061892
- PMCID: PMC5665807
- DOI: 10.1098/rstb.2016.0419
The origin of heredity in protocells
Abstract
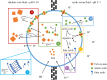
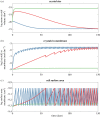
Here we develop a computational model that examines one of the first major biological innovations-the origin of heredity in simple protocells. The model assumes that the earliest protocells were autotrophic, producing organic matter from CO2 and H2 Carbon fixation was facilitated by geologically sustained proton gradients across fatty acid membranes, via iron-sulfur nanocrystals lodged within the membranes. Thermodynamic models suggest that organics formed this way should include amino acids and fatty acids. We assume that fatty acids partition to the membrane. Some hydrophobic amino acids chelate FeS nanocrystals, producing three positive feedbacks: (i) an increase in catalytic surface area; (ii) partitioning of FeS nanocrystals to the membrane; and (iii) a proton-motive active site for carbon fixing that mimics the enzyme Ech. These positive feedbacks enable the fastest-growing protocells to dominate the early ecosystem through a simple form of heredity. We propose that as new organics are produced inside the protocells, the localized high-energy environment is more likely to form ribonucleotides, linking RNA replication to its ability to drive protocell growth from the beginning. Our novel conceptualization sets out conditions under which protocell heredity and competition could arise, and points to where crucial experimental work is required.This article is part of the themed issue 'Process and pattern in innovations from cells to societies'.
Keywords: RNA world; composome; origin of life; protocell.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
-
- Woese CR. 1967. The genetic code: the molecular basis for genetic expression. New York, NY: Harper & Row.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
