Canonical NFκB signaling in myeloid cells is required for the glioblastoma growth
- PMID: 29062041
- PMCID: PMC5653749
- DOI: 10.1038/s41598-017-14079-4
Canonical NFκB signaling in myeloid cells is required for the glioblastoma growth
Abstract
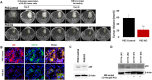
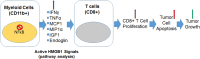
Tumor development and therapeutic resistance are linked with tumor-associated macrophage (TAM) and myeloid-derived suppressor cell (MDSC) infiltration in tumors via chemokine axis. Chemokine expression, which determines the pro or anti-inflammatory status of myeloid cells, are partly regulated by the nuclear factor-kappa B (NF-κB) pathway. Here, we identified that conditional deletion of canonical NF-κB signaling (p65) in myeloid cells inhibited syngeneic glioblastoma (GBM) through decreased CD45 infiltration in tumors, as characterized by decreased TAMs (CD206+) and MDSCs (Gr1+ CD11b+), increased dendritic cells (CD86+) and cytotoxic T cells (CD8+) in the p65 knockout (KO) mice. Proinflammatory cytokines (IFNγ, MCP1, MIP1α, and TNFα) and myeloid differentiation factor (Endoglin) were increased in myeloid cells from p65 KO tumor, which demonstrated an influence on CD8+T cell proliferation. In contrast, p65KO athymic chimeric mice with human GBM, failed to inhibit tumor growth, confirming the contribution of T cells in an immune competent model. The analysis of human datasets and GBM tumors revealed higher expression of p65 in GBM-associated CD68+ macrophages compared to neighboring stroma. Thus, canonical NF-κB signaling has an anti-inflammatory role and is required for macrophage polarization, immune suppression, and GBM growth. Combining an NF-κB inhibitor with standard therapy could improve antitumor immunity in GBM.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Lahmar Q, et al. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochimica et biophysica acta. 2016;1865:23–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

