Recycled iron fuels new production in the eastern equatorial Pacific Ocean
- PMID: 29062103
- PMCID: PMC5653654
- DOI: 10.1038/s41467-017-01219-7
Recycled iron fuels new production in the eastern equatorial Pacific Ocean
Erratum in
-
Publisher Correction: Recycled iron fuels new production in the eastern equatorial Pacific Ocean.Nat Commun. 2017 Dec 5;8(1):2018. doi: 10.1038/s41467-017-02138-3. Nat Commun. 2017. PMID: 29209057 Free PMC article.
Abstract
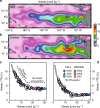
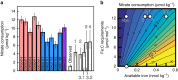
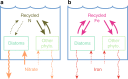
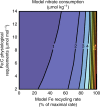
Nitrate persists in eastern equatorial Pacific surface waters because phytoplankton growth fueled by nitrate (new production) is limited by iron. Nitrate isotope measurements provide a new constraint on the controls of surface nitrate concentration in this region and allow us to quantify the degree and temporal variability of nitrate consumption. Here we show that nitrate consumption in these waters cannot be fueled solely by the external supply of iron to these waters, which occurs by upwelling and dust deposition. Rather, a substantial fraction of nitrate consumption must be supported by the recycling of iron within surface waters. Given plausible iron recycling rates, seasonal variability in nitrate concentration on and off the equator can be explained by upwelling rate, with slower upwelling allowing for more cycles of iron regeneration and uptake. The efficiency of iron recycling in the equatorial Pacific implies the evolution of ecosystem-level mechanisms for retaining iron in surface ocean settings where it limits productivity.
Conflict of interest statement
The authors have no competing financial interests.
Figures

Similar articles
-
No iron fertilization in the equatorial Pacific Ocean during the last ice age.Nature. 2016 Jan 28;529(7587):519-22. doi: 10.1038/nature16453. Nature. 2016. PMID: 26819045
-
Ocean dynamics, not dust, have controlled equatorial Pacific productivity over the past 500,000 years.Proc Natl Acad Sci U S A. 2016 May 31;113(22):6119-24. doi: 10.1073/pnas.1600616113. Epub 2016 May 16. Proc Natl Acad Sci U S A. 2016. PMID: 27185933 Free PMC article.
-
A mesoscale iron enrichment in the western subarctic Pacific induces a large centric diatom bloom.Science. 2003 May 9;300(5621):958-61. doi: 10.1126/science.1082000. Science. 2003. PMID: 12738858
-
Iron, phytoplankton growth, and the carbon cycle.Met Ions Biol Syst. 2005;43:153-93. doi: 10.1201/9780824751999.ch7. Met Ions Biol Syst. 2005. PMID: 16370118 Review.
-
Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions.Science. 2007 Feb 2;315(5812):612-7. doi: 10.1126/science.1131669. Science. 2007. PMID: 17272712 Review.
Cited by
-
Probing the Bioavailability of Dissolved Iron to Marine Eukaryotic Phytoplankton Using In Situ Single Cell Iron Quotas.Global Biogeochem Cycles. 2021 Aug;35(8):e2021GB006979. doi: 10.1029/2021GB006979. Epub 2021 Aug 25. Global Biogeochem Cycles. 2021. PMID: 35865367 Free PMC article.
-
Tropical decadal variability in nutrient supply and phytoplankton community in the Central Equatorial Pacific during the late Holocene.Sci Rep. 2024 Feb 20;14(1):4161. doi: 10.1038/s41598-024-54635-3. Sci Rep. 2024. PMID: 38378879 Free PMC article.
-
Ocean iron cycle feedbacks decouple atmospheric CO2 from meridional overturning circulation changes.Nat Commun. 2024 Jul 8;15(1):5532. doi: 10.1038/s41467-024-49274-1. Nat Commun. 2024. PMID: 38977666 Free PMC article.
-
Biogeochemical feedbacks associated with the response of micronutrient recycling by zooplankton to climate change.Glob Chang Biol. 2021 Oct;27(19):4758-4770. doi: 10.1111/gcb.15789. Epub 2021 Jul 29. Glob Chang Biol. 2021. PMID: 34228873 Free PMC article.
-
The influence of Arctic Fe and Atlantic fixed N on summertime primary production in Fram Strait, North Greenland Sea.Sci Rep. 2020 Sep 17;10(1):15230. doi: 10.1038/s41598-020-72100-9. Sci Rep. 2020. PMID: 32943713 Free PMC article.
References
-
- Dugdale RC, Goering JJ. Uptake of new and regenerated forms of nitrogen in primary production. Limnol. Oceanogr. 1967;12:196–206. doi: 10.4319/lo.1967.12.2.0196. - DOI
-
- Martin, J. H. Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanography5, 1–13 (1990).
-
- Hutchins DA, DiTullio GR, Bruland KW. Iron and regenerated production: evidence for biological iron recycling in two environments. Limnol. Oceanogr. 1993;38:1242–1255. doi: 10.4319/lo.1993.38.6.1242. - DOI
-
- Landry MR, et al. Iron and grazing constraints on primary production in the central equatorial Pacific: an EqPac synthesis. Limnol. Oceanogr. 1997;42:405–418. doi: 10.4319/lo.1997.42.3.0405. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

