Enhancing the efficacy of AREDS antioxidants in light-induced retinal degeneration
- PMID: 29062223
- PMCID: PMC5640517
Enhancing the efficacy of AREDS antioxidants in light-induced retinal degeneration
Abstract
Purpose: Light-induced photoreceptor cell degeneration and disease progression in age-related macular degeneration (AMD) involve oxidative stress and visual cell loss, which can be prevented, or slowed, by antioxidants. Our goal was to test the protective efficacy of a traditional Age-related Eye Disease Study antioxidant formulation (AREDS) and AREDS combined with non-traditional antioxidants in a preclinical animal model of photooxidative retinal damage.
Methods: Male Sprague-Dawley rats were reared in a low-intensity (20 lux) or high-intensity (200 lux) cyclic light environment for 6 weeks. Some animals received a daily dietary supplement consisting of a small cracker infused with an AREDS antioxidant mineral mixture, AREDS antioxidants minus zinc, or zinc oxide alone. Other rats received AREDS combined with a detergent extract of the common herb rosemary, AREDS plus carnosic acid, zinc oxide plus rosemary, or rosemary alone. Antioxidant efficacy was determined by measuring retinal DNA levels 2 weeks after 6 h of intense exposure to white light (9,000 lux). Western blotting was used to determine visual cell opsin and arrestin levels following intense light treatment. Rhodopsin regeneration was determined after 1 h of exposure to light. Gene array analysis was used to determine changes in the expression of retinal genes resulting from light rearing environment or from antioxidant supplementation.
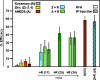
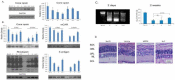
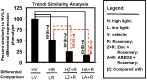
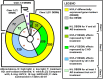
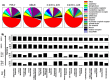
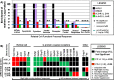
Results: Chronic high-intensity cyclic light rearing resulted in lower levels of rod and cone opsins, retinal S-antigen (S-ag), and medium wavelength cone arrestin (mCAR) than found for rats maintained in low cyclic light. However, as determined by retinal DNA, and by residual opsin and arrestin levels, 2 weeks after acute photooxidative damage, visual cell loss was greater in rats reared in low cyclic light. Retinal damage decreased with AREDS plus rosemary, or with zinc oxide plus rosemary whereas AREDS alone and zinc oxide alone (at their daily recommended levels) were both ineffective. One week of supplemental AREDS plus carnosic acid resulted in higher levels of rod and cone cell proteins, and higher levels of retinal DNA than for AREDS alone. Rhodopsin regeneration was unaffected by the rosemary treatment. Retinal gene array analysis showed reduced expression of medium- wavelength opsin 1 and arrestin C in the high-light reared rats versus the low-light rats. The transition of rats from low cyclic light to a high cyclic light environment resulted in the differential expression of 280 gene markers, enriched for genes related to inflammation, apoptosis, cytokine, innate immune response, and receptors. Rosemary, zinc oxide plus rosemary, and AREDS plus rosemary suppressed 131, 241, and 266 of these genes (respectively) in high-light versus low-light animals and induced a small subset of changes in gene expression that were independent of light rearing conditions.
Conclusions: Long-term environmental light intensity is a major determinant of retinal gene and protein expression, and of visual cell survival following acute photooxidative insult. Rats preconditioned by high-light rearing exhibit lower levels of cone opsin mRNA and protein, and lower mCAR protein, than low-light reared animals, but greater retention of retinal DNA and proteins following photooxidative damage. Rosemary enhanced the protective efficacy of AREDS and led to the greatest effect on the retinal genome in animals reared in high environmental light. Chronic administration of rosemary antioxidants may be a useful adjunct to the therapeutic benefit of AREDS in slowing disease progression in AMD.
Figures
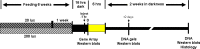


Similar articles
-
Prevention of retinal light damage by zinc oxide combined with rosemary extract.Mol Vis. 2013 Jun 27;19:1433-45. Print 2013. Mol Vis. 2013. PMID: 23825923 Free PMC article.
-
Light history and age-related changes in retinal light damage.Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1107-16. Invest Ophthalmol Vis Sci. 1998. PMID: 9620069
-
Delayed loss of cone and remaining rod photoreceptor cells due to impairment of choroidal circulation after acute light exposure in rats.Invest Ophthalmol Vis Sci. 2007 Apr;48(4):1864-72. doi: 10.1167/iovs.06-1065. Invest Ophthalmol Vis Sci. 2007. PMID: 17389522
-
Retinal light damage: mechanisms and protection.Prog Retin Eye Res. 2010 Mar;29(2):113-34. doi: 10.1016/j.preteyeres.2009.11.004. Epub 2009 Dec 3. Prog Retin Eye Res. 2010. PMID: 19951742 Free PMC article. Review.
-
Who should receive oral supplement treatment for age-related macular degeneration?Curr Opin Ophthalmol. 2003 Jun;14(3):159-62. doi: 10.1097/00055735-200306000-00009. Curr Opin Ophthalmol. 2003. PMID: 12777936 Review.
Cited by
-
Zinc and Autophagy in Age-Related Macular Degeneration.Int J Mol Sci. 2020 Jul 15;21(14):4994. doi: 10.3390/ijms21144994. Int J Mol Sci. 2020. PMID: 32679798 Free PMC article. Review.
-
NUTRARET: Effect of 2-Year Nutraceutical Supplementation on Redox Status and Visual Function of Patients With Retinitis Pigmentosa: A Randomized, Double-Blind, Placebo-Controlled Trial.Front Nutr. 2022 Mar 21;9:847910. doi: 10.3389/fnut.2022.847910. eCollection 2022. Front Nutr. 2022. PMID: 35387197 Free PMC article.
-
Overexpression of ß-Arrestin1 in the Rostral Ventrolateral Medulla Downregulates Angiotensin Receptor and Lowers Blood Pressure in Hypertension.Front Physiol. 2018 Mar 28;9:297. doi: 10.3389/fphys.2018.00297. eCollection 2018. Front Physiol. 2018. PMID: 29643817 Free PMC article.
-
Retinal Neurodegeneration: Correlation between Nutraceutical Treatment and Animal Model.Nutrients. 2021 Feb 27;13(3):770. doi: 10.3390/nu13030770. Nutrients. 2021. PMID: 33673449 Free PMC article.
-
Zinc deficiency as possible link between immunosenescence and age-related diseases.Immun Ageing. 2025 May 19;22(1):19. doi: 10.1186/s12979-025-00511-1. Immun Ageing. 2025. PMID: 40390089 Free PMC article. Review.
References
-
- Eye Disease Case-Control Study Group Risk factors for neovascular age-related macular degeneration. Arch Ophthalmol. 1992;110:1701–8. - PubMed
-
- Zarbin M, Sunness JS. Dry age-related macular degeneration and age-related macular degeneration pathogenesis. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. London: Saunders Elsevier; 2010. p. 527–535.
-
- Lederer DE, Cousins SW, Csaky KG. Neovascular age-related macular degeneration. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. London: Saunders Elsevier; 2010. p. 536–543.
-
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, Yannuzzi LA, Willett W, Eye Disease Case-Control Study Group Dietary carotenoids, vitamin A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272:1413–20. - PubMed
-
- Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM. Risk factors for age-related macular degeneration. European Ophthalmic Rev. 2011;5:143–53.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

