pH-Dependant Antifungal Activity of Valproic Acid against the Human Fungal Pathogen Candida albicans
- PMID: 29062309
- PMCID: PMC5640775
- DOI: 10.3389/fmicb.2017.01956
pH-Dependant Antifungal Activity of Valproic Acid against the Human Fungal Pathogen Candida albicans
Abstract
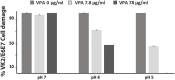
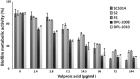
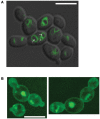
Current antifungal drugs suffer from limitations including toxicity, the emergence of resistance and decreased efficacy at low pH that are typical of human vaginal surfaces. Here, we have shown that the antipsychotic drug valproic acid (VPA) exhibited a strong antifungal activity against both sensitive and resistant Candida albicans in pH condition similar to that encountered in vagina. VPA exerted a strong anti-biofilm activity and attenuated damage of vaginal epithelial cells caused by C. albicans. We also showed that VPA synergizes with the allylamine antifungal, Terbinafine. We undertook a chemogenetic screen to delineate biological processes that underlies VPA-sensitivity in C. albicans and found that vacuole-related genes were required to tolerate VPA. Confocal fluorescence live-cell imaging revealed that VPA alters vacuole integrity and support a model where alteration of vacuoles contributes to the antifungal activity. Taken together, this study suggests that VPA could be used as an effective antifungal against vulvovaginal candidiasis.
Keywords: Candida albicans; antifungal; vacuole; valproic acid; vulvovaginal candidiasis.
Figures

Similar articles
-
ERG2 and ERG24 Are Required for Normal Vacuolar Physiology as Well as Candida albicans Pathogenicity in a Murine Model of Disseminated but Not Vaginal Candidiasis.Eukaryot Cell. 2015 Oct;14(10):1006-16. doi: 10.1128/EC.00116-15. Epub 2015 Jul 31. Eukaryot Cell. 2015. PMID: 26231054 Free PMC article.
-
Prevalence and antifungal susceptibility of Candida albicans and its related species Candida dubliniensis and Candida africana isolated from vulvovaginal samples in a hospital of Argentina.Rev Argent Microbiol. 2016 Jan-Mar;48(1):43-9. doi: 10.1016/j.ram.2015.10.003. Epub 2016 Feb 26. Rev Argent Microbiol. 2016. PMID: 26922471
-
In vitro synergistic activity of ketoconazole with valproic acid against Candida species.Arzneimittelforschung. 1996 Sep;46(9):934-6. Arzneimittelforschung. 1996. PMID: 8876946
-
Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery.Int J Antimicrob Agents. 2014 May;43(5):395-402. doi: 10.1016/j.ijantimicag.2013.12.009. Epub 2014 Jan 22. Int J Antimicrob Agents. 2014. PMID: 24503221 Review.
-
Antifungal drug resistance in pathogenic fungi.Med Mycol. 1998;36 Suppl 1:119-28. Med Mycol. 1998. PMID: 9988500 Review.
Cited by
-
Molecular Tools for Targeted Control of Nerve Cell Electrical Activity. Part II.Acta Naturae. 2021 Oct-Dec;13(4):17-32. doi: 10.32607/actanaturae.11415. Acta Naturae. 2021. PMID: 35127143 Free PMC article.
-
Antifungal Activity of Bulgarian Rose Damascena Oil against Vaginitis-Causing Opportunistic Fungi.Evid Based Complement Alternat Med. 2023 Dec 1;2023:5054865. doi: 10.1155/2023/5054865. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38074845 Free PMC article.
-
Antifungal Efficacy of Redox-Active Natamycin against Some Foodborne Fungi-Comparison with Aspergillus fumigatus.Foods. 2021 Sep 2;10(9):2073. doi: 10.3390/foods10092073. Foods. 2021. PMID: 34574183 Free PMC article.
-
Anti-Fungal Potential of Structurally Diverse FDA-Approved Therapeutics Targeting Secreted Aspartyl Proteinase (SAP) of Candida albicans: an In Silico Drug Repurposing Approach.Appl Biochem Biotechnol. 2023 Mar;195(3):1983-1998. doi: 10.1007/s12010-022-04207-w. Epub 2022 Nov 19. Appl Biochem Biotechnol. 2023. PMID: 36401722
-
RAFT-Derived Polymethacrylates as a Superior Treatment for Recurrent Vulvovaginal Candidiasis by Targeting Biotic Biofilms and Persister Cells.Front Microbiol. 2019 Nov 7;10:2592. doi: 10.3389/fmicb.2019.02592. eCollection 2019. Front Microbiol. 2019. PMID: 31787962 Free PMC article.
References
-
- Clinical and Laboratory Standards Institute (2008). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard M27-A3, 3rd Edn. Approved Standard M27-A3 (Wayne, PA: CLSI; ).
LinkOut - more resources
Full Text Sources
Other Literature Sources

