Alcohol Inhibits Odontogenic Differentiation of Human Dental Pulp Cells by Activating mTOR Signaling
- PMID: 29062364
- PMCID: PMC5618757
- DOI: 10.1155/2017/8717454
Alcohol Inhibits Odontogenic Differentiation of Human Dental Pulp Cells by Activating mTOR Signaling
Abstract
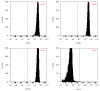
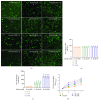
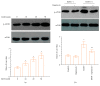
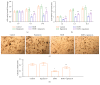
Long-term heavy alcohol consumption could result in a range of health, social, and behavioral problems. People who abuse alcohol are at high risks of seriously having osteopenia, periodontal disease, and compromised oral health. However, the role of ethanol (EtOH) in the biological functions of human dental pulp cells (DPCs) is unknown. Whether EtOH affects the odontoblastic differentiation of DPCs through the mechanistic target of rapamycin (mTOR) remains unexplored. The objective of this study was to investigate the effects of EtOH on DPC differentiation and mineralization. DPCs were isolated and purified from human dental pulps. The proliferation and odontoblastic differentiation of DPCs treated with EtOH were subsequently investigated. Different doses of EtOH were shown to be cytocompatible with DPCs. EtOH significantly activated the mTOR pathway in a dose-dependent manner. In addition, EtOH downregulated the alkaline phosphatase activity, attenuated the mineralized nodule formation, and suppressed the expression of odontoblastic markers including ALP, DSPP, DMP-1, Runx2, and OCN. Moreover, the pretreatment with rapamycin, a specific mTOR inhibitor, markedly reversed the EtOH-induced odontoblastic differentiation and cell mineralization. Our findings show for the first time that EtOH can suppress DPC differentiation and mineralization in a mTOR-dependent manner, indicating that EtOH may be involved in negatively regulating the dental pulp repair.
Figures
Similar articles
-
Metformin Enhances the Differentiation of Dental Pulp Cells into Odontoblasts by Activating AMPK Signaling.J Endod. 2018 Apr;44(4):576-584. doi: 10.1016/j.joen.2017.11.017. Epub 2018 Jan 4. J Endod. 2018. PMID: 29306537 Free PMC article.
-
Effects of leukemia inhibitory factor on proliferation and odontoblastic differentiation of human dental pulp cells.J Endod. 2011 Jun;37(6):819-24. doi: 10.1016/j.joen.2011.02.031. Epub 2011 May 6. J Endod. 2011. PMID: 21787496
-
Concentrated Growth Factor Promotes Dental Pulp Cells Proliferation and Mineralization and Facilitates Recovery of Dental Pulp Tissue.Med Sci Monit. 2019 Dec 26;25:10016-10028. doi: 10.12659/MSM.919316. Med Sci Monit. 2019. PMID: 31877561 Free PMC article.
-
Simvastatin and nanofibrous poly(l-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment.Acta Biomater. 2018 Mar 1;68:190-203. doi: 10.1016/j.actbio.2017.12.037. Epub 2017 Dec 30. Acta Biomater. 2018. PMID: 29294374 Free PMC article.
-
Effects of WNT10A on proliferation and differentiation of human dental pulp cells.J Endod. 2014 Oct;40(10):1593-9. doi: 10.1016/j.joen.2014.07.009. Epub 2014 Aug 16. J Endod. 2014. PMID: 25134734
Cited by
-
Stem cells under the influence of alcohol: effects of ethanol consumption on stem/progenitor cells.Cell Mol Life Sci. 2019 Jan;76(2):231-244. doi: 10.1007/s00018-018-2931-8. Epub 2018 Oct 10. Cell Mol Life Sci. 2019. PMID: 30306211 Free PMC article. Review.
-
Dental-Derived Mesenchymal Stem Cells: State of the Art.Front Cell Dev Biol. 2021 Jun 22;9:654559. doi: 10.3389/fcell.2021.654559. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34239870 Free PMC article. Review.
-
Novel Calcium Phosphate Cement with Metformin-Loaded Chitosan for Odontogenic Differentiation of Human Dental Pulp Cells.Stem Cells Int. 2018 Nov 27;2018:7173481. doi: 10.1155/2018/7173481. eCollection 2018. Stem Cells Int. 2018. PMID: 30598667 Free PMC article.
-
Effect of two different concentrations of 1α,25-dihydroxyvitamin D3 on odontogenic differentiation of stem cells from human exfoliated deciduous teeth.Dent Res J (Isfahan). 2022 Jan 28;19:4. doi: 10.4103/1735-3327.336689. eCollection 2022. Dent Res J (Isfahan). 2022. PMID: 35308448 Free PMC article.
-
A multi-organ analysis of the role of mTOR in fetal alcohol spectrum disorders.FASEB J. 2023 May;37(5):e22897. doi: 10.1096/fj.202201865R. FASEB J. 2023. PMID: 37000494 Free PMC article. Review.
References
-
- Turner R. T. Skeletal response to alcohol. Alcoholism, Clinical and Experimental Research. 2000;24(11):1693–1701. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

