Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance
- PMID: 29062489
- PMCID: PMC5648753
- DOI: 10.1038/s41522-017-0035-0
Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance
Abstract
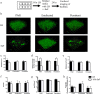
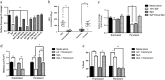
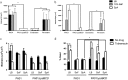
Antimicrobial resistance is a significant threat to the treatment of infectious disease. Multiple mechanisms of resistance to different classes of antibiotics have been identified and well-studied. However, these mechanisms are studied with bacteria in isolation, whereas often, infections have a polymicrobial basis. Using a biofilm slide chamber model, we visualized the formation and development of clinical Pseudomonas aeruginosa biofilms in the presence of secreted Staphylococcus aureus exoproducts, two bacteria that commonly co-infect pediatric patients with cystic fibrosis. We showed that, over time, certain isolates of P. aeruginosa can form different biofilm architecture in the presence of S. aureus exoproducts. We further determined that this interaction was dependent on Psl produced by P. aeruginosa and staphylococcal protein A from S. aureus. Importantly, we identified a mechanism of antibiotic resistance to tobramycin that is dependent on the polymicrobial interactions between these two bacteria. This interaction occurred in isolates of P. aeruginosa recovered from children with cystic fibrosis who failed to clear P. aeruginosa following inhaled tobramycin treatment.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



References
-
- O’Neill, J. Antimicrobial resistance: tackling a crisis for health and wealth of nations. Rev. Antimicrob. Resistance 16 (2014).
-
- Doern G, Breacher S. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J. Clin. Microbiol. 2011;49:S11. doi: 10.1128/JCM.00580-11. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

