"Cut and Sew" Transformations via Transition-Metal-Catalyzed Carbon-Carbon Bond Activation
- PMID: 29062586
- PMCID: PMC5650109
- DOI: 10.1021/acscatal.6b03210
"Cut and Sew" Transformations via Transition-Metal-Catalyzed Carbon-Carbon Bond Activation
Abstract
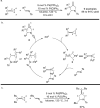
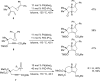
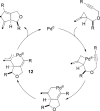
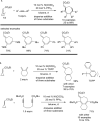
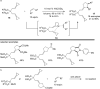
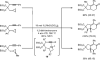
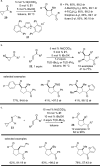
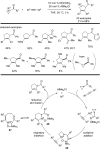
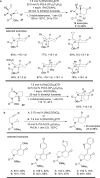
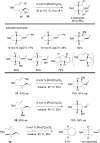
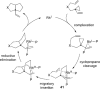
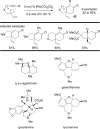
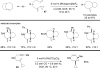
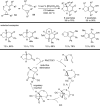
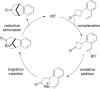
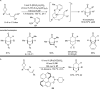
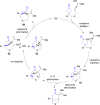
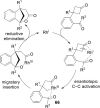
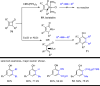
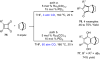
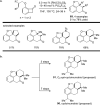
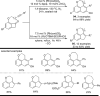
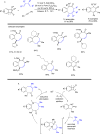
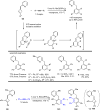
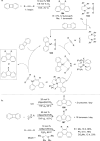
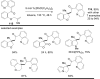
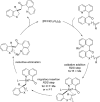
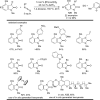
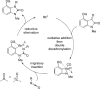
The transition metal-catalyzed "cut and sew" transformation has recently emerged as a useful strategy for preparing complex molecular structures. After oxidative addition of a transition metal into a carbon-carbon bond, the resulting two carbon termini can be both functionalized in one step via the following migratory insertion and reductive elimination with unsaturated units, such as alkenes, alkynes, allenes, CO and polar multiple bonds. Three- or four-membered rings are often employed as reaction partners due to their high ring strains. The participation of non-strained structures generally relies on cleavage of a polar carbon-CN bond or assistance of a directing group.
Keywords: carbon–carbon activation; cut and sew; oxidative addition; reductive elimination; transition-metal catalysis.
Figures

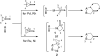









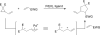







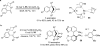



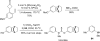





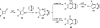









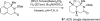






References
-
- Murakami M, Ito Y. In: Activation of Unreactive Bonds and Organic Synthesis. Topics in Organometallic Chemistry. Murai S, editor. Vol. 3. Springer; Berlin: 1999. pp. 97–129.
-
- Dong G, editor. C-C Bond Activation. Topics in Current Chemistry. Vol. 346 Springer; Berlin: 2014. - PubMed
- Souillart L, Cramer N. Chem. Rev. 2015;115:9410–9464. - PubMed
- Rybtchinski B, Milstein D. Angew. Chem. Int. Ed. 1999;38:870–883. - PubMed
- Perthuisot C, Edelbach BL, Zubris DL, Simhai N, Iverson CN, Müller C, Satoh T, Jones WD. J. Mol. Catal. A: Chem. 2002;189:157–168.
- Ruhland K. Eur. J. Org. Chem. 2012;2012:2683–2706.
- Chen F, Wang T, Jiao N. Chem. Rev. 2014;114:8613–8661. - PubMed
- Dermenci A, Coe JW, Dong G. Org. Chem. Front. 2014;1:567–581. - PMC - PubMed
- Kondo T, Mitsudo T-a. Chem. Lett. 2005;34:1462–1467.
- Teruyuki K, Take-aki M. Chem. Lett. 2005;34:1462–1467.
- Jones WD. Nature. 1993;364:676–677.
- Murakami M, Matsuda T. Chem. Commun. 2011;47:1100–1105. - PubMed
- Jun C-H. Chem. Soc. Rev. 2004;33:610–618. - PubMed
- Murakami M, Amii H, Ito Y. Nature. 1994;370:540–541.
-
- Tsuji J, editor. Palladium in Organic Synthesis. Topics in Organometallic Chemistry. Vol. 14 Springer; Berlin: 2005.
- Cramer N, Seiser T. Synlett. 2011;2011:449–460.
-
- Bellus D, Ernst B. Angew. Chem. Int. Ed. 1988;27:797–827.
- Namyslo JC, Kaufmann DE. Chem. Rev. 2003;103:1485–1538. - PubMed
- Sadana AK, Saini RK, Billups WE. Chem. Rev. 2003;103:1539–1602. - PubMed
- Seiser T, Saget T, Tran DN, Cramer N. Angew. Chem. Int. Ed. 2011;50:7740–7752. - PubMed
- Belluš D, Ernst B. Angew. Chem. Int. Ed. 1988;27:797–827.
- Flores-Gaspar A, Martin R. Synthesis. 2013;45:563–580.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
