PaCER - A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation
- PMID: 29062684
- PMCID: PMC5645007
- DOI: 10.1016/j.nicl.2017.10.004
PaCER - A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation
Abstract
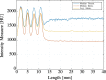
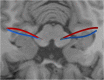
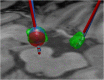
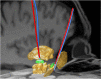
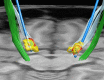
Deep brain stimulation (DBS) is a neurosurgical intervention where electrodes are permanently implanted into the brain in order to modulate pathologic neural activity. The post-operative reconstruction of the DBS electrodes is important for an efficient stimulation parameter tuning. A major limitation of existing approaches for electrode reconstruction from post-operative imaging that prevents the clinical routine use is that they are manual or semi-automatic, and thus both time-consuming and subjective. Moreover, the existing methods rely on a simplified model of a straight line electrode trajectory, rather than the more realistic curved trajectory. The main contribution of this paper is that for the first time we present a highly accurate and fully automated method for electrode reconstruction that considers curved trajectories. The robustness of our proposed method is demonstrated using a multi-center clinical dataset consisting of N = 44 electrodes. In all cases the electrode trajectories were successfully identified and reconstructed. In addition, the accuracy is demonstrated quantitatively using a high-accuracy phantom with known ground truth. In the phantom experiment, the method could detect individual electrode contacts with high accuracy and the trajectory reconstruction reached an error level below 100 μm (0.046 ± 0.025 mm). An implementation of the method is made publicly available such that it can directly be used by researchers or clinicians. This constitutes an important step towards future integration of lead reconstruction into standard clinical care.
Figures







References
-
- Akram H., Sotiropoulos S.N., Jbabdi S., Georgiev D., Mahlknecht P., Hyam J., Foltynie T., Limousin P., De Vita E., Jahanshahi M., Hariz M., Ashburner J., Behrens T., Zrinzo L. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in parkinson's disease. NeuroImage. 2017;158:332–345. (Jul.) - PMC - PubMed
-
- Castrioto A., Lhommée E., Moro E., Krack P. Mood and behavioural effects of subthalamic stimulation in parkinson's disease. Lancet Neurol. 2014;13:287–305. (Mar.) - PubMed
-
- Chen L., Hallmann M., Romero D., Wang L., Astrom M., Ryzhkov M., Nijlunsing R., Meine H. Combining tubular tracking and skeletonization for fully-automatic and accurate lead localization in CT images. Int. J. Comput. Assist. Radiol. Surg. 2014;9(1):S195–S201. (may)
-
- Coenen V.A., Allert N., Paus S., Kronenbürger M., Urbach H., Mädler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery. 2014;75:657–669. (discussion 669-70, Dec.) - PubMed
-
- da Silva N.M., Rozanski V.E., Cunha J.P.S. Proc. 7th Int. IEEE/EMBS Conf. Neural Engineering (NER) 2015. A 3D multimodal approach to precisely locate dbs electrodes in the basal ganglia brain region; pp. 292–295. (Apr.)
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

