Metabolic advantages and vulnerabilities in brain metastases
- PMID: 29063238
- PMCID: PMC5712254
- DOI: 10.1007/s10585-017-9864-8
Metabolic advantages and vulnerabilities in brain metastases
Abstract
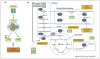
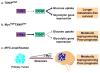
Metabolic adaptations permit tumor cells to metastasize to and thrive in the brain. Brain metastases continue to present clinical challenges due to rising incidence and resistance to current treatments. Therefore, elucidating altered metabolic pathways in brain metastases may provide new therapeutic targets for the treatment of aggressive disease. Due to the high demand for glucose in the brain, increased glycolytic activity is favored for energy production. Primary tumors that undergo Warburg-like metabolic reprogramming become suited to growth in the brain microenvironment. Indeed, elevated metabolism is a predictor of metastasis in many cancer subtypes. Specifically, metabolic alterations are seen in primary tumors that are associated with the formation of brain metastases, namely breast cancer, lung cancer, and melanoma. Because of this selective pressure, inhibitors of key metabolic factors may reduce tumor cell viability, thus exploiting metabolic pathways for cancer therapeutics. This review summarizes the metabolic advantages and vulnerabilities of brain metastases.
Keywords: Brain metastases; Cancer metabolism; Metabolic adaptation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nature reviews. Cancer. 2006;6:449–58. - PubMed
-
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Current oncology reports. 2012;14:48–54. - PubMed
-
- Markesbery WR, Brooks WH, Gupta GD, Young AB. Treatment for patients with cerebral metastases. Archives of neurology. 1978;35:754–6. - PubMed
-
- Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:419–25. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

