Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study
- PMID: 29063242
- PMCID: PMC5688213
- DOI: 10.1007/s00415-017-8635-4
Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study
Abstract
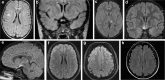
Anti-myelin oligodendrocyte glycoprotein antibodies (MOG-Ab) recently emerged as a potential biomarker in patients with inflammatory demyelinating diseases of the central nervous system. We here compare the clinical and laboratory findings observed in a cohort of MOG-Ab seropositive and seronegative cases and describe IgG subclass analysis results. Consecutive serum samples referred to Verona University Neuropathology Laboratory for aquaporin-4 (AQP4)-Ab and/or MOG-Ab testing were analysed between March 2014 and May 2017. The presence of AQP4-Ab was determined using a cell-based assay. A live cell immunofluorescence assay was used for the detection of MOG-IgG and IgG subclass analysis. Among 454 analysed samples, 29 were excluded due to AQP4-Ab positivity or to the final demonstration of a disorder not compatible with MOG-Ab. We obtained clinical data in 154 out of 425 cases. Of these, 22 subjects resulted MOG-Ab positive. MOG-Ab positive patients were mainly characterised by the involvement of the optic nerve and/or spinal cord. Half of the cases presented relapses and the recovery was usually partial. Brain MRI was heterogeneous while short lesions were the prevalent observation on spinal cord MRI. MOG-Ab titre usually decreased in non-relapsing cases. In all MOG-IgG positive cases, we observed IgG1 antibodies, which were predominant in most subjects. IgG2 (5/22), IgG3 (9/22) and IgG4 (3/22) antibodies were also detectable. We confirm that MOG-Ab-related syndromes have distinct features in the spectrum of demyelinating conditions, and we describe the possible role of the different IgG subclasses in this condition.
Keywords: Acute disseminated encephalomyelitis (ADEM); Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies; Multiple sclerosis (MS); Myelitis; Neuromyelitis optica spectrum disorders (NMOSD); Optic neuritis.
Conflict of interest statement
Conflicts of interest
Dr. Capra received lecture fees from Novartis, Biogen, Teva, Genzyme and Sanofi-Aventis. M. Reindl is an academic editor for PLoS One. The University Hospital, and Medical University of Innsbruck (Austria, Markus Reindl) receives payments for antibody assays (NMDAR, AQP4, and other autoantibodies) and for MOG and AQP4 antibody validation experiments organized by Euroimmun (Lübeck, Germany). Dr. Gajofatto received travel support to attend scientific meeting by Almirall, Biogen, Merck, and Novartis. The other authors declare that they have no conflict of interest.
Ethical standards
All human studies have been performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and its later amendments.
Informed consent
All patients consented to diagnostic procedures and biological sample storage at Verona Neuropathology Laboratory.
Funding
Sara Mariotto is currently supported by a research fellowship of the European Academy of Neurology. Markus Reindl is supported by a research grants from the Austrian Federal Ministry of Science and Economy (Grant BIG WIG MS, Markus Reindl) and the Austrian Research promotion Agency (FFG, Bridge 1 Project No. 853209 EDNA).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

