Efficacy of fingolimod and interferon beta-1b on cognitive, MRI, and clinical outcomes in relapsing-remitting multiple sclerosis: an 18-month, open-label, rater-blinded, randomised, multicentre study (the GOLDEN study)
- PMID: 29063244
- PMCID: PMC5688215
- DOI: 10.1007/s00415-017-8642-5
Efficacy of fingolimod and interferon beta-1b on cognitive, MRI, and clinical outcomes in relapsing-remitting multiple sclerosis: an 18-month, open-label, rater-blinded, randomised, multicentre study (the GOLDEN study)
Abstract
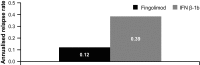
Cognitive impairment (CI) affects 40-65% of multiple sclerosis (MS) patients. This study attempted evaluating the effects of fingolimod and interferon beta-1b (IFN β-1b) on CI progression, magnetic resonance imaging (MRI) and clinical outcomes in relapsing-remitting MS (RRMS) patients over 18 months. The GOLDEN study was a pilot study including RRMS patients with CI randomised (2:1) to fingolimod (0.5 mg daily)/IFN β-1b (250 µg every other day). CI was assessed via Rao's Brief Repeatable Battery and Delis-Kaplan Executive Function System test. MRI parameters, Expanded Disability Status Scale scores and relapses were measured. Overall, 157 patients were randomised, of whom 30 discontinued the study (fingolimod, 8.49%; IFN β-1b, 41.18%; p ≤ 0.0001). Patients randomised to fingolimod had more severe clinical and MRI disease characteristics at baseline compared with IFN β-1b. At Month (M) 18, both treatment groups showed improvements in all cognitive parameters. At M18, relapse rate, total number and volume of T2/T1 gadolinium-enhancing lesions were higher with IFN β-1b, as well as the percentage brain volume change during the study. Safety and tolerability of both treatments were similar to previous studies. Both treatments showed improvements in cognitive parameters. Fingolimod demonstrated significantly better effects on MRI parameters and relapse rate. Imbalance in baseline characteristics and the drop-out pattern may have favoured IFN β-1b. A longer duration trial may be needed to observe the complete expression of differential effects on CI scales reflecting the between-groups differences on MRI. Although limited in size, the GOLDEN study confirms the favourable benefit-risk profile of fingolimod reported in previous studies.
Keywords: Brain atrophy; Brief repeatable battery test; Cognitive impairment; Delis–Kaplan executive function test; Fingolimod; Interferon beta-1b.
Conflict of interest statement
Conflicts of interest
G Comi has received compensation for consulting services and/or speaking activities from Novartis, Teva, Sanofi, Genzyme, Merck, Biogen, Roche, Almirall, Celgene, Forward Pharma, Excemed. F. Patti received speaking honoraria and fee for advisory board activities by Amirall, Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi-Genzyme and TEVA; he also received research grant by MIUR and Fondazione Italiana Sclerosi Multipla. M. Rocca received speakers honoraria from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva and Merk Serono and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. M. Filippi is Editor-in-Chief of the Journal of Neurology; serves on a scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Biogen Idec, Merk-Serono, Novartis, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer’s Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and ARiSLA (Fondazione Italiana di Ricerca per la SLA). F.Mattioli received travel and conference fees and editorial consulence fees from Merck Serono, Biogen and Novartis. M. P. Amato received research grants and honoraria as a speaker and member of advisory boards by Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Teva, Almirall. P. Gallo has been a consultant for Bayer Schering, Biogen Idec, Genzyme, Merck Serono and Novartis, has received funding for travel and speaker honoraria from Merck-Serono, Biogen Idec, Sanofi-Aventis, Novartis Pharma and Bayer-Schering Pharma, Teva, has received research support from Bayer, Biogen Idec/Elan, MerkSerono, Genzyme and Teva, and has received research grant from the University of Padova, Veneto Region of Italy, the Italian Association for Multiple Sclerosis, the Italian Ministry of Public Health. D. Centonze is an advisory board member of Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck-Serono, Novartis, Sanofi-Genzyme, Teva and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen Idec, GW Pharmaceuticals, Merck Serono, Novartis, Sanofi-Genzyme, Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen Idec, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, Teva. His preclinical and clinical research was supported by grants from Bayer, Biogen, Merck Serono, Novartis and Teva. C. Pozzilli has served on scientific advisory boards for Actelion, Biogen, Genzyme, Hoffmann-La Roche Ltd,Merck-Serono, Novartis, Sanofi, Teva, and has received consulting and/or speaking fees, research support and travel grants from Allergan, Almirall, Biogen, Genzyme, Hoffmann-La Roche Ltd, Merck-Serono, Novartis, Sanofi and Teva. F. Saccà received personal compensation from Novartis, Almirall, Genzyme, Biogen, Merck Serono Forward Pharma and TEVA for public speaking, editorial work and advisory boards. F. Then Bergh has received research support for investigator-initiated studies and support for local scientific symposia (to the University of Leipzig), travel support for scientific meetings, and personal compensation as a speaker or serving on advisory boards from Actelion, Bayer-Schering, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Sanofi-Aventis, TEVA. He has received public research funding from the Deutsche Forschungsgemeinschaft, the German Ministry of Education and Research and the Myelinprojekt. He has no direct financial interest in products or procedures described in this work or employed in the study reported. M. Bartezaghi and R. Turrini are employees of Novartis Farma.
Ethical standards
All patients provided written informed consent before enrolment. The study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Funding
The study was funded by Novartis Farma, Origgio, Varese, Italy.
Figures





References
-
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O’Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stuve O, Waubant E, Polman CH. Defining the clinical course of multiple sclerosis The 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

