Canine invasive mammary carcinomas as models of human breast cancer. Part 2: immunophenotypes and prognostic significance
- PMID: 29063312
- PMCID: PMC5790838
- DOI: 10.1007/s10549-017-4542-8
Canine invasive mammary carcinomas as models of human breast cancer. Part 2: immunophenotypes and prognostic significance
Abstract
Purpose: Relevant animal models of human breast cancer are currently needed, especially for the aggressive triple-negative breast cancer subtype. Recent studies and our results (Part 1) indicate that spontaneous canine invasive mammary carcinomas (CMCs) resemble human breast cancer by clinics and pathology as well as behavior and prognostic indicators. We hypothesized that the current molecular classifications of human breast cancer, used for therapeutic decision, could be relevant to dogs.
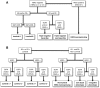
Methods: Three hundred and fifty female dogs with spontaneous CMC and a 2-year follow-up were retrospectively included. By immunohistochemistry, CMCs were classified according to Nielsen (Clin Cancer Res 10:5367-5374, 2004) and Blows (PlosOne doi: 10.1371/journal.pmed.1000279, 2010) into the subtypes of human breast cancer.
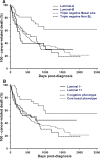
Results: Four immunophenotypes were defined either according to Nielsen classification (luminal A 14.3%, luminal B 9.4%, triple-negative basal-like 58.6%, and triple-negative nonbasal-like 17.7% CMCs); or to Blows classification (luminal 1-: 11.4%, luminal 1+: 12.3%, Core basal phenotype: 58.6%, and five-negative phenotype: 17.7%). No HER2-overexpressing CMC as defined by a 3 + immunohistochemical score was observed in our cohort. By univariate and multivariate analyses, both immunophenotypical classifications applied to CMCs showed strong prognostic significance: luminal A or luminal 1+ CMCs showed a significantly longer disease-free interval (HR = 0.46), Overall (HR = 0.47), and Specific Survival (HR = 0.56) compared to triple-negative carcinomas, after adjustment for stage.
Conclusions: In our cohort, triple-negative CMCs largely predominated (76%), were much more prevalent than in human beings, and showed an aggressive natural behavior after mastectomy. Dogs are thus potent valuable spontaneous models to test new therapeutic strategies for this particular subtype of breast cancer.
Keywords: Animal model; Breast cancer; Dog; Immunophenotype; Luminal; Triple-negative.
Conflict of interest statement
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The owners’ written consent and approval from the Oniris College of Veterinary Medicine local Animal Welfare Committee were obtained prior to inclusion of each canine mammary carcinoma in this retrospective observational study.
Figures
References
-
- NHS Cancer Screening Programmes and The Royal College of Pathologists (2005) Pathology Reporting of Breast Disease: A Joint Document Incorporating the Third Edition of the NHS Breast Screening Programme’s Guidelines for Pathology Reporting in Breast Cancer Screening and the Second Edition of The Royal College of Pathologists’ Minimum Dataset for Breast Cancer Histopathology. Sheffield. https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil.... Accessed 5 Nov 2016
-
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

