Directed Evolution: Bringing New Chemistry to Life
- PMID: 29064156
- PMCID: PMC5901037
- DOI: 10.1002/anie.201708408
Directed Evolution: Bringing New Chemistry to Life
Abstract
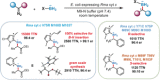
Tailor-made: Discussed herein is the ability to adapt biology's mechanisms for innovation and optimization to solving problems in chemistry and engineering. The evolution of nature's enzymes can lead to the discovery of new reactivity, transformations not known in biology, and reactivity inaccessible by small-molecule catalysts.
Keywords: biocatalysis; enzymes; heme proteins; protein engineering; synthetic methods.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

