Deterioration of Limb Muscle Function during Acute Exacerbation of Chronic Obstructive Pulmonary Disease
- PMID: 29064260
- PMCID: PMC5821903
- DOI: 10.1164/rccm.201703-0615CI
Deterioration of Limb Muscle Function during Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Abstract
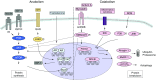
Important features of both stable and acute exacerbation of chronic obstructive pulmonary disease (COPD) are skeletal muscle weakness and wasting. Limb muscle dysfunction during an exacerbation has been linked to various adverse outcomes, including prolonged hospitalization, readmission, and mortality. The contributing factors leading to muscle dysfunction are similar to those seen in stable COPD: disuse, nutrition/energy balance, hypercapnia, hypoxemia, electrolyte derangements, inflammation, and drugs (i.e., glucocorticoids). These factors may be the trigger for a downstream cascade of local inflammatory changes, pathway process alterations, and structural degradation. Ultimately, the clinical effects can be wide ranging and include reduced limb muscle strength. Current therapies, such as pulmonary/physical rehabilitation, have limited impact because of low participation rates. Recently, novel drugs have been developed in similar disorders, and learnings from these studies can be used as a foundation to facilitate discovery in patients hospitalized with a COPD exacerbation. Nevertheless, investigators should approach this patient population with knowledge of the limitations of each intervention. In this Concise Clinical Review, we provide an overview of acute muscle dysfunction in patients hospitalized with acute exacerbation of COPD and a strategic approach to drug development in this setting.
Keywords: COPD; drug development; exacerbations; pharmacological interventions; skeletal muscle.
Figures

References
-
- Jones SE, Maddocks M, Kon SS, Canavan JL, Nolan CM, Clark AL, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70:213–218. - PubMed
-
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. - PMC - PubMed
-
- Vilaró J, Ramirez-Sarmiento A, Martínez-Llorens JM, Mendoza T, Alvarez M, Sánchez-Cayado N, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med. 2010;104:1896–1902. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

