Zinc Signals and Immunity
- PMID: 29064429
- PMCID: PMC5666901
- DOI: 10.3390/ijms18102222
Zinc Signals and Immunity
Abstract
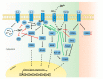
Zinc homeostasis is crucial for an adequate function of the immune system. Zinc deficiency as well as zinc excess result in severe disturbances in immune cell numbers and activities, which can result in increased susceptibility to infections and development of especially inflammatory diseases. This review focuses on the role of zinc in regulating intracellular signaling pathways in innate as well as adaptive immune cells. Main underlying molecular mechanisms and targets affected by altered zinc homeostasis, including kinases, caspases, phosphatases, and phosphodiesterases, will be highlighted in this article. In addition, the interplay of zinc homeostasis and the redox metabolism in affecting intracellular signaling will be emphasized. Key signaling pathways will be described in detail for the different cell types of the immune system. In this, effects of fast zinc flux, taking place within a few seconds to minutes will be distinguish from slower types of zinc signals, also designated as "zinc waves", and late homeostatic zinc signals regarding prolonged changes in intracellular zinc.
Keywords: homeostatic zinc signal; immune function; innate and adaptive immunity; signaling pathways; zinc deficiency; zinc flux; zinc wave.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Prasad A.S., Miale A., Jr., Farid Z., Sandstead H.H., Schulert A.R. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J. Lab. Clin. Med. 1963;61:537–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

