Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites
- PMID: 29064452
- PMCID: PMC5666435
- DOI: 10.3390/md15100329
Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites
Abstract
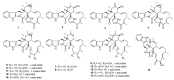
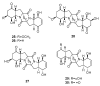
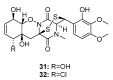
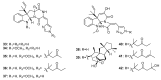
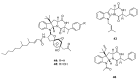
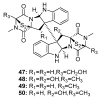
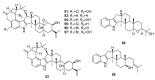
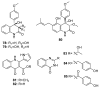
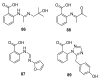
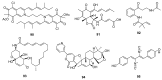
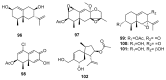
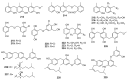
Since the discovery of penicillin, Penicillium has become one of the most attractive fungal genera for the production of bioactive molecules. Marine-derived Penicillium has provided numerous excellent pharmaceutical leads over the past decades. In this review, we focused on the cytotoxic metabolites * (* Cytotoxic potency was referred to five different levels in this review, extraordinary (IC50/LD50: <1 μM or 0.5 μg/mL); significant (IC50/LD50: 1~10 μM or 0.5~5 μg/mL); moderate (IC50/LD50: 10~30 μM or 5~15 μg/mL); mild (IC50/LD50: 30~50 μM or 15~25 μg/mL); weak (IC50/LD50: 50~100 μM or 25~50 μg/mL). The comparative potencies of positive controls were referred when they were available). produced by marine-derived Penicillium species, and on their cytotoxicity mechanisms, biosyntheses, and chemical syntheses.
Keywords: biosynthesis; cytotoxic metabolites; marine-derived Penicillium; natural products.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
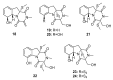

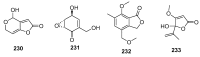
Similar articles
-
The Cytotoxic Activity of Secondary Metabolites from Marine-Derived Penicillium spp.: A Review (2018-2024).Mar Drugs. 2025 Apr 30;23(5):197. doi: 10.3390/md23050197. Mar Drugs. 2025. PMID: 40422787 Free PMC article. Review.
-
Bioactive polyketides and alkaloids from Penicillium citrinum, a fungal endophyte isolated from Ocimum tenuiflorum.Fitoterapia. 2013 Dec;91:100-106. doi: 10.1016/j.fitote.2013.08.017. Epub 2013 Aug 31. Fitoterapia. 2013. PMID: 23999155
-
Origins, Structures, and Bioactivities of Secondary Metabolites from Marine-derived Penicillium Fungi.Mini Rev Med Chem. 2021;21(15):2000-2019. doi: 10.2174/1389557521666210217093517. Mini Rev Med Chem. 2021. PMID: 33596801
-
Novel Bioactive Natural Products from Marine-Derived Penicillium Fungi: A Review (2021-2023).Mar Drugs. 2024 Apr 23;22(5):191. doi: 10.3390/md22050191. Mar Drugs. 2024. PMID: 38786582 Free PMC article. Review.
-
Recent Advances in the Isolation, Synthesis and Biological Activity of Marine Guanidine Alkaloids.Mar Drugs. 2017 Oct 24;15(10):324. doi: 10.3390/md15100324. Mar Drugs. 2017. PMID: 29064383 Free PMC article. Review.
Cited by
-
Chromosome-Level Comprehensive Genome of Mangrove Sediment-Derived Fungus Penicillium variabile HXQ-H-1.J Fungi (Basel). 2019 Dec 23;6(1):7. doi: 10.3390/jof6010007. J Fungi (Basel). 2019. PMID: 31878043 Free PMC article.
-
The Mycobiota of the Deep Sea: What Omics Can Offer.Life (Basel). 2020 Nov 19;10(11):292. doi: 10.3390/life10110292. Life (Basel). 2020. PMID: 33228036 Free PMC article. Review.
-
Characterization of Drug-like Chemical Space for Cytotoxic Marine Metabolites Using Multivariate Methods.ACS Omega. 2019 Mar 31;4(3):5402-5411. doi: 10.1021/acsomega.8b01764. Epub 2019 Mar 18. ACS Omega. 2019. PMID: 31179404 Free PMC article.
-
Anti-Inflammatory Azaphilones from the Edible Alga-Derived Fungus Penicillium sclerotiorum.Mar Drugs. 2021 Sep 22;19(10):529. doi: 10.3390/md19100529. Mar Drugs. 2021. PMID: 34677428 Free PMC article.
-
Molecular networking as a tool to annotate the metabolites of Bacillus sp. and Serratia marcescens isolates and evaluate their fungicidal effects against Magnapothe oryzae and Bipolaris oryzae.3 Biotech. 2023 May;13(5):148. doi: 10.1007/s13205-023-03547-6. Epub 2023 Apr 28. 3 Biotech. 2023. PMID: 37128476 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

