Post-Translational Modifications of Protein Backbones: Unique Functions, Mechanisms, and Challenges
- PMID: 29064683
- PMCID: PMC5770884
- DOI: 10.1021/acs.biochem.7b00861
Post-Translational Modifications of Protein Backbones: Unique Functions, Mechanisms, and Challenges
Abstract
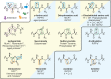
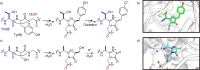
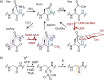
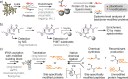
Post-translational modifications (PTMs) dramatically enhance the capabilities of proteins. They introduce new functionalities and dynamically control protein activity by modulating intra- and intermolecular interactions. Traditionally, PTMs have been considered as reversible attachments to nucleophilic functional groups on amino acid side chains, whereas the polypeptide backbone is often thought to be inert. This paradigm is shifting as chemically and functionally diverse alterations of the protein backbone are discovered. Importantly, backbone PTMs can control protein structure and function just as side chain modifications do and operate through unique mechanisms to achieve these features. In this Perspective, I outline the various types of protein backbone modifications discovered so far and highlight their contributions to biology as well as the challenges in studying this versatile yet poorly characterized class of PTMs.
Conflict of interest statement
The author declares no competing financial interest.
Figures

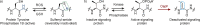

References
-
- Walsh C. (2006) Posttranslational modification of proteins: Expanding nature’s inventory, Roberts and Company Publishers, Greenwood, CO.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

