Senescence-associated ultrastructural features of long-term cultures of induced pluripotent stem cells (iPSCs)
- PMID: 29064821
- PMCID: PMC5680563
- DOI: 10.18632/aging.101309
Senescence-associated ultrastructural features of long-term cultures of induced pluripotent stem cells (iPSCs)
Abstract
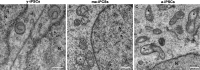
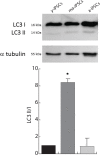
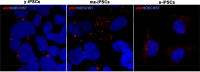
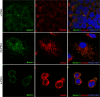
Induced pluripotent stem cells (iPSCs) hold great promise for developing personalized regenerative medicine, however characterization of their biological features is still incomplete. Moreover, changes occurring in long-term cultured iPSCs have been reported, suggesting these as a model of cellular aging. For this reason, we addressed the ultrastructural characterization of iPSCs, with a focus on possible time-dependent changes, involving specific cell compartments. To this aim, we comparatively analysed cultures at different timepoints, by an innovative electron microscopic technology (FIB/SEM). We observed progressive loss of cell-to-cell contacts, associated with increased occurrence of exosomes. Mitochondria gradually increased, while acquiring an elongated shape, with well-developed cristae. Such mitochondrial maturation was accompanied by their turnover, as assessed by the presence of autophagomes (undetectable in young iPSCs), some containing recognizable mitochondria. This finding was especially frequent in middle-aged iPSCs, while being occasional in aged cells, suggesting early autophagic activation followed by a decreased efficiency of the process with culturing time. Accordingly, confocal microscopy showed age-dependent alterations to the expression and distribution of autophagic markers. Interestingly, responsivity to rapamycin, highest in young iPSCs, was almost lost in aged cells. Overall, our results strongly support long-term cultured iPSCs as a model for studying relevant aspects of cellular senescence, involving intercellular communication, energy metabolism, and autophagy.
Keywords: FIB/SEM; aging; autophagy; cell-cell contacts; iPSCs; mitochondria.
Conflict of interest statement
The authors of this manuscript declare no conflict of interests.
Figures





References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. https://doi.org/10.1016/j.cell.2006.07.024 - DOI - PubMed
-
- Compagnucci C, Nizzardo M, Corti S, Zanni G, Bertini E. In vitro neurogenesis: development and functional implications of iPSC technology. Cell Mol Life Sci. 2014;71:1623–39. https://doi.org/10.1007/s00018-013-1511-1 - DOI - PMC - PubMed
-
- Bukowiecki R, Adjaye J, Prigione A. Mitochondrial function in pluripotent stem cells and cellular reprogramming. Gerontology. 2014;60:174–82. https://doi.org/10.1159/000355050 - DOI - PubMed
-
- Raikwar SP, Kim EM, Sivitz WI, Allamargot C, Thedens DR, Zavazava N. Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice. PLoS One. 2015;10:e0116582. https://doi.org/10.1371/journal.pone.0116582 - DOI - PMC - PubMed
-
- Beckmann A, Schubert M, Hainz N, Haase A, Martin U, Tschernig T, Meier C. Ultrastructural demonstration of Cx43 gap junctions in induced pluripotent stem cells from human cord blood. Histochem Cell Biol. 2016;146:529–37. https://doi.org/10.1007/s00418-016-1469-9 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

