Risk stratification of childhood cancer survivors necessary for evidence-based clinical long-term follow-up
- PMID: 29065109
- PMCID: PMC5729444
- DOI: 10.1038/bjc.2017.347
Risk stratification of childhood cancer survivors necessary for evidence-based clinical long-term follow-up
Abstract
Background: Reorganisation of clinical follow-up care in England was proposed by the National Cancer Survivorship Initiative (NCSI), based on cancer type and treatment, ranging from Level 1 (supported self-management) to Level 3 (consultant-led care). The objective of this study was to provide an investigation of the risks of serious adverse health-outcomes associated with NCSI Levels of clinical care using a large population-based cohort of childhood cancer survivors.
Methods: The British Childhood Cancer Survivor Study (BCCSS) was used to investigate risks of specific causes of death, subsequent primary neoplasms (SPNs) and non-fatal non-neoplastic outcomes by NCSI Level.
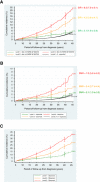
Results: Cumulative (excess) risks of specified adverse outcomes by 45 years from diagnosis among non-leukaemic survivors assigned to NCSI Levels 1, 2 and 3 were for: SPNs-5% (two-fold expected), 14% (four-fold expected) and 21% (eight-fold expected); non-neoplastic death-2% (two-fold expected), 4% (three-fold expected) and 8% (seven-fold expected); non-fatal non-neoplastic condition-14%, 27% and 40%, respectively. Consequently overall cumulative risks of any adverse health outcome were 21%, 45% and 69%, respectively.
Conclusions: Despite its simplicity the risk stratification tool provides clear and strong discrimination between survivors assigned to different NCSI Levels in terms of long-term cumulative and excess risks of serious adverse outcomes.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form and declare: Dr Frobisher, Dr Lancashire, Dr Reulen, Professor Hawkins, Mr Winter and Ms Kelly reports grants from Department of Health, England, grants from Cancer Research UK, grants from Kay Kendall Leukaemia Fund, grants from PanCareSurFup, European 7th Framework Programme; Dr Glaser reports he was Clinical Director of the National Cancer Survivor Initiative at the Department of Health, England between 2010 and 2013; the remaining authors have no conflict of interest.
Figures


References
-
- Absolom K, Greenfield D, Ross R, Horne B, Davies H, Glaser A, Simpson A, Waite H, Eiser C (2006) Predictors of clinic satisfaction among adult survivors of childhood cancer. Eur J Cancer 42: 1421–1427. - PubMed
-
- Cancer Therapy Evaluation Programme (2006) Common Terminology Criteria for Adverse events. Version 3.0. National Cancer Institute: Bethesda, MD. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/... (accessed 19 August 2015).
-
- Children's Oncology Group (COG) (2013) Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancer. Version 4.0. Available at: http://www.survivorshipguidelines.org/ (accessed 5 March 2016).
-
- Coviello V, Bogges M (2004) Cumulative incidence estimation in the presence of competing risks. Stata J 4(2): 103–112.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

