Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required?
- PMID: 29065110
- PMCID: PMC5729478
- DOI: 10.1038/bjc.2017.367
Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required?
Abstract
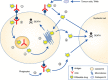
Antibody drug conjugates (ADCs) employ the exquisite specificity of tumour-specific monoclonal antibodies (mAb) for the targeted delivery of highly potent cytotoxic drugs to the tumour site. The chemistry of the linker, which connects the drug to the mAb, determines how and when the drug is released from the mAb. This, as well as the chemistry of the drug, can dictate whether the drug can diffuse into surrounding cells, resulting in 'bystander killing'. Initially, any bystander killing mechanism of action of an ADC was understood to involve an essential sequence of steps beginning with surface antigen targeting, internalisation, intracellular linker cleavage, drug release, and diffusion of drug away from the targeted cell. However, recent studies indicate that, depending on the linker and drug combination, this mechanism may not be essential and ADCs can be cleaved extracellularly or via other mechanisms. In this minireview, we will examine the role of bystander killing by ADCs and explore the emerging evidence of how this can occur independently of internalisation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ansell SM (2014) Brentuximab vedotin. Blood 124(22): 3197–3200. - PubMed
-
- Bartlett NL, Sharman JP, Oki Y, Advani RH, Bello CM, Winter JN, Yang Y, Kennedy DA, Jacobsen ED (2013) A phase 2 study Of brentuximab vedotin in patients with relapsed or refractory CD30-positive non-Hodgkin lymphomas: interim results in patients with DLBCL and other B-Cell lymphomas. Blood 122(21): 848.
-
- Bartlett NL, Smith MR, Siddiqi T, Advani RH, O'Connor OA, Sharman JP, Feldman T, Savage KJ, Shustov AR, Diefenbach CS, Oki Y, Palanca-Wessels MC, Uttarwar M, Li M, Yang J, Jacobsen ED (2017) Brentuximab vedotin activity in diffuse large B-cell lymphoma with CD30 undetectable by visual assessment of conventional immunohistochemistry. Leuk Lymphoma 58(7): 1607–1616. - PubMed
-
- Beck A, Goetsch L, Dumontet C, Corvaia N (2017) Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 16(5): 315–337. - PubMed
-
- Bernardes GJ, Casi G, Trussel S, Hartmann I, Schwager K, Scheuermann J, Neri D (2012) A traceless vascular-targeting antibody-drug conjugate for cancer therapy. Angew Chem Int Ed Engl 51(4): 941–944. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

