Concomitant nevirapine impacts pharmacokinetic exposure to the antimalarial artemether-lumefantrine in African children
- PMID: 29065172
- PMCID: PMC5655345
- DOI: 10.1371/journal.pone.0186589
Concomitant nevirapine impacts pharmacokinetic exposure to the antimalarial artemether-lumefantrine in African children
Abstract
Background: The antiretroviral drug nevirapine and the antimalarial artemisinin-based combination therapy artemether-lumefantrine are commonly co-administered to treat malaria in the context of HIV. Nevirapine is a known inhibitor of cytochrome P450 3A4, which metabolizes artemether and lumefantrine. To address the concern that the antiretroviral nevirapine impacts the antimalarial artemether-lumefantrine pharmacokinetics, a prospective non-randomized controlled study in children presenting with uncomplicated malaria and HIV in sub-Saharan Africa was carried out.
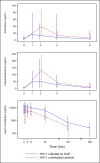
Methods: Participants received artemether-lumefantrine (20/120 mg weight-based BID) for 3 days during nevirapine-based antiretroviral therapy (ART) co-administration (158-266 mg/m2 QD). HIV positive participants who were not yet on ART drugs were also enrolled as the control group. The target enrollment was children aged 3-12 years (n = 24 in each group). Intensive pharmacokinetics after the last artemether-lumefantrine dose was assessed for artemether, its active metabolite dihydroartemisinin, and lumefantrine. Pharmacokinetic parameters (area under the plasma concentration vs. time curve (AUC), maximum concentration and day 7 lumefantrine concentrations) were estimated using non-compartmental methods and compared to controls.
Results: Nineteen children (16 on nevirapine and three not on ART) enrolled. Fifteen of the 16 (aged 4 to 11 years) on nevirapine-based ART were included in the pharmacokinetic analysis. Due to evolving WHO HIV treatment guidelines, insufficient children were enrolled in the control group (n = 3), so the pharmacokinetic data were compared to a historical control group of 20 HIV-uninfected children 5-12 years of age who also presented with malaria and underwent identical study procedures. Decreases of pharmacokinetic exposure [as estimated by AUC (AUC0-8hr)] were marginally significant for artemether (by -46%, p = 0.08) and dihydroartemisinin (-22%, p = 0.06) in the children on nevirapine-based ART, compared to when artemether-lumefantrine was administered alone. Similarly, peak concentration was decreased by 50% (p = 0.07) for artemether and 36% (p = 0.01) for dihydroartemisinin. In contrast, exposure to lumefantrine increased significantly in the context of nevirapine [AUC0-120hr:123% (p<0.001); Cday7:116% (p<0.001), Cmax: 95% (p<0.001)].
Conclusions: Nevirapine-based ART increases the exposure to lumefantrine in pre-pubescent children with a trend toward diminished artemether and dihydroartemisinin exposure. These findings contrast with other studies indicating NVP reduces or results in no change in exposure of antimalarial drugs, and may be specific to this age group (4-12 years). Considering the excellent safety profile of artemether-lumefantrine, the increase in lumefantrine is not of concern. However, the reduction in artemisinin exposure may warrant further study, and suggests that dosage adjustment of artemether-lumefantrine with nevirapine-based ART in children is likely warranted.
Conflict of interest statement
Figures
References
-
- Malaria biology. [Internet]. [cited November 23, 2016]. Available from: http://www.cdc.gov/malaria/about/biology/index.html.
-
- World Malaria Report 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/wmr201.... Accessed on December 28, 2015. [Internet]. 2015.
-
- HIV/AIDS, Data and Statistics. http://www.who.int/hiv/data/en/. Accessed on December 28, 2015. [Internet].
-
- Makanga M, Krudsood S. The clinical efficacy of artemether/lumefantrine (Coartem). Malaria journal. 2009;8 Suppl 1:S5 doi: 10.1186/1475-2875-8-S1-S5 ; PubMed Central PMCID: PMCPMC2760240. - DOI - PMC - PubMed
-
- WHO. Antiretroviral therapy for HIV infection in infants and children: Towards universal access. Recommendations for a public health approach, 2010 revision. World Health Organization website: http://apps.who.int/iris/bitstream/10665/164255/1/9789241599801_eng.pdf?...; accessed on August 30, 2017. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

