Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21
- PMID: 29065221
- PMCID: PMC5758394
- DOI: 10.1111/bph.14079
Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21
Abstract
Background and purpose: Berberine, a compound from rhizome coptidis, is traditionally used to treat gastrointestinal infections, such as bacterial diarrhoea. Recently, berberine was shown to have hypoglycaemic and hypolipidaemic effects. We investigated the mechanisms by which berberine regulates hepatic lipid metabolism and energy expenditure in mice.
Experimental approach: Liver-specific SIRT1 knockout mice and their wild-type littermates were fed a high-fat, high-sucrose (HFHS) diet and treated with berberine by i.p. injection for five weeks. Mouse primary hepatocytes and human HepG2 cells were treated with berberine and then subjected to immunoblotting analysis and Oil Red O staining.
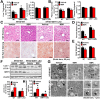
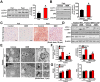
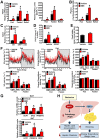
Key results: Berberine attenuated hepatic steatosis and controlled energy balance in mice by inducing autophagy and FGF21. These beneficial effects of berberine on autophagy and hepatic steatosis were abolished by a deficiency of the nutrient sensor SIRT1 in the liver of HFHS diet-fed obese mice and in mouse primary hepatocytes. SIRT1 is essential for berberine to potentiate autophagy and inhibit lipid storage in mouse livers in response to fasting. Mechanistically, the berberine stimulates SIRT1 deacetylation activity and induces autophagy in an autophagy protein 5-dependent manner. Moreover, the administration of berberine was shown to promote hepatic gene expression and circulating levels of FGF21 and ketone bodies in mice in a SIRT1-dependent manner.
Conclusions and implications: Berberine acts in the liver to regulate lipid utilization and maintain whole-body energy metabolism by mediating autophagy and FGF21 activation. Hence, it has therapeutic potential for treating metabolic defects under nutritional overload, such as fatty liver diseases, type 2 diabetes and obesity.
© 2017 The British Pharmacological Society.
Figures




References
-
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos‐Flier E (2007). Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

