Cinnamic Acid Analogs as Intervention Catalysts for Overcoming Antifungal Tolerance
- PMID: 29065462
- PMCID: PMC6151797
- DOI: 10.3390/molecules22101783
Cinnamic Acid Analogs as Intervention Catalysts for Overcoming Antifungal Tolerance
Abstract
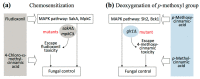
Disruption of fungal cell wall should be an effective intervention strategy. However, the cell wall-disrupting echinocandin drugs, such as caspofungin (CAS), cannot exterminate filamentous fungal pathogens during treatment. For potency improvement of cell wall-disrupting agents (CAS, octyl gallate (OG)), antifungal efficacy of thirty-three cinnamic acid derivatives was investigated against Saccharomyces cerevisiaeslt2Δ, bck1Δ, mutants of the mitogen-activated protein kinase (MAPK), and MAPK kinase kinase, respectively, in cell wall integrity system, and glr1Δ, mutant of CAS-responsive glutathione reductase. Cell wall mutants were highly susceptible to four cinnamic acids (4-chloro-α-methyl-, 4-methoxy-, 4-methyl-, 3-methylcinnamic acids), where 4-chloro-α-methyl- and 4-methylcinnamic acids possessed the highest activity. Structure-activity relationship revealed that 4-methylcinnamic acid, the deoxygenated structure of 4-methoxycinnamic acid, overcame tolerance of glr1Δ to 4-methoxycinnamic acid, indicating the significance of para substitution of methyl moiety for effective fungal control. The potential of compounds as chemosensitizers (intervention catalysts) to cell wall disruptants (viz., 4-chloro-α-methyl- or 4-methylcinnamic acids + CAS or OG) was assessed according to Clinical Laboratory Standards Institute M38-A. Synergistic chemosensitization greatly lowers minimum inhibitory concentrations of the co-administered drug/agents. 4-Chloro-α-methylcinnamic acid further overcame fludioxonil tolerance of Aspergillus fumigatus antioxidant MAPK mutants (sakAΔ, mpkCΔ). Collectively, 4-chloro-α-methyl- and 4-methylcinnamic acids possess chemosensitizing capability to augment antifungal efficacy of conventional drug/agents, thus could be developed as target-based (i.e., cell wall disruption) intervention catalysts.
Keywords: antifungal; antioxidant system; caspofungin; cell wall integrity; chemosensitization; cinnamic acids; intervention catalysts; small molecules; synergism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Augmenting the Activity of Monoterpenoid Phenols against Fungal Pathogens Using 2-Hydroxy-4-methoxybenzaldehyde that Target Cell Wall Integrity.Int J Mol Sci. 2015 Nov 10;16(11):26850-70. doi: 10.3390/ijms161125988. Int J Mol Sci. 2015. PMID: 26569223 Free PMC article.
-
Antifungal activity of redox-active benzaldehydes that target cellular antioxidation.Ann Clin Microbiol Antimicrob. 2011 May 31;10:23. doi: 10.1186/1476-0711-10-23. Ann Clin Microbiol Antimicrob. 2011. PMID: 21627838 Free PMC article.
-
Puupehenone, a Marine-Sponge-Derived Sesquiterpene Quinone, Potentiates the Antifungal Drug Caspofungin by Disrupting Hsp90 Activity and the Cell Wall Integrity Pathway.mSphere. 2020 Jan 8;5(1):e00818-19. doi: 10.1128/mSphere.00818-19. mSphere. 2020. PMID: 31915228 Free PMC article.
-
Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin.Med Mycol. 2005 May;43 Suppl 1:S299-305. doi: 10.1080/13693780400029023. Med Mycol. 2005. PMID: 16110824 Review.
-
Caspofungin: an echinocandin antifungal agent.Clin Ther. 2002 Mar;24(3):351-77; discussion 329. doi: 10.1016/s0149-2918(02)85039-1. Clin Ther. 2002. PMID: 11952021 Review.
Cited by
-
Antifungal Drug Repurposing.Antibiotics (Basel). 2020 Nov 15;9(11):812. doi: 10.3390/antibiotics9110812. Antibiotics (Basel). 2020. PMID: 33203147 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

