Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine
- PMID: 29065466
- PMCID: PMC5666890
- DOI: 10.3390/ijms18102210
Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine
Abstract
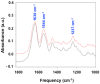
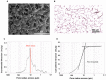
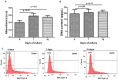
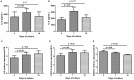
Maintenance of mesenchymal stem cells (MSCs) requires a tissue-specific microenvironment (i.e., niche), which is poorly represented by the typical plastic substrate used for two-dimensional growth of MSCs in a tissue culture flask. The objective of this study was to address the potential use of collagen-based medical devices (HEMOCOLLAGENE®, Saint-Maur-des-Fossés, France) as mimetic niche for MSCs with the ability to preserve human MSC stemness in vitro. With a chemical composition similar to type I collagen, HEMOCOLLAGENE® foam presented a porous and interconnected structure (>90%) and a relative low elastic modulus of around 60 kPa. Biological studies revealed an apparently inert microenvironment of HEMOCOLLAGENE® foam, where 80% of cultured human MSCs remained viable, adopted a flattened morphology, and maintained their undifferentiated state with basal secretory activity. Thus, three-dimensional HEMOCOLLAGENE® foams present an in vitro model that mimics the MSC niche with the capacity to support viable and quiescent MSCs within a low stiffness collagen I scaffold simulating Wharton's jelly. These results suggest that haemostatic foam may be a useful and versatile carrier for MSC transplantation for regenerative medicine applications.
Keywords: biocompatibility; medical device; paracrine activities; regenerative medicine; stem cell niche.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Smith J.R., Pfeifer K., Petry F., Powell N., Delzeit J., Weiss M.L. Standardizing umbilical cord mesenchymal stromal cells for translation to clinical use: Selection of GMP-compliant medium and a simplified isolation method. Stem Cells Int. 2016;2016:e6810980. doi: 10.1155/2016/6810980. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

