A SOA-Based Platform to Support Clinical Data Sharing
- PMID: 29065576
- PMCID: PMC5463102
- DOI: 10.1155/2017/2190679
A SOA-Based Platform to Support Clinical Data Sharing
Abstract
The eSource Data Interchange Group, part of the Clinical Data Interchange Standards Consortium, proposed five scenarios to guide stakeholders in the development of solutions for the capture of eSource data. The fifth scenario was subdivided into four tiers to adapt the functionality of electronic health records to support clinical research. In order to develop a system belonging to the "Interoperable" Tier, the authors decided to adopt the service-oriented architecture paradigm to support technical interoperability, Health Level Seven Version 3 messages combined with LOINC (Logical Observation Identifiers Names and Codes) vocabulary to ensure semantic interoperability, and Healthcare Services Specification Project standards to provide process interoperability. The developed architecture enhances the integration between patient-care practice and medical research, allowing clinical data sharing between two hospital information systems and four clinical data management systems/clinical registries. The core is formed by a set of standardized cloud services connected through standardized interfaces, involving client applications. The system was approved by a medical staff, since it reduces the workload for the management of clinical trials. Although this architecture can realize the "Interoperable" Tier, the current solution actually covers the "Connected" Tier, due to local hospital policy restrictions.
Figures


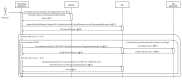
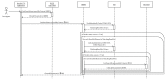


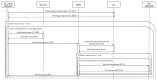

References
-
- Altman D. G. Practical Statistics for Medical Research. Chapman and Hall: CRC Press; 1990.
-
- Gerritsen M. G., Sartorius O. E., vd Veen F. M., Meester G. T. Data management in multi-center clinical trials and the role of a nation-wide computer network. A 5 year evaluation. Proceedings of the Annual Symposium on Computer Application in Medical Care; 1993; Washington, DC. pp. 659–662. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

